Synthesis,Structure and Properties of Ultra-high Molecular Weight Poly(1-octene) as high efficient Drag Reducer for Oil Pipeline Transportation (Part 1)
Abstract
Pipeline transportation has the advantages of low cost and plays an important role in oil transportation. According to the report "2020 China Oil and Gas Pipeline Construction and New Progress", the total mileage of oil pipeline transportation in China has reached nearly 6×105 km, pipeline transportation is related to China's energy security issues.
Long chains of 1-octene with a molecular weight of up to millions α- Olefin polymers can maintain excellent drag reduction effects for a long time and can be used as drag reducing agents for petroleum pipeline transportation. The core technology for developing ultra-high molecular weight poly (1-octene) is the design and optimization of catalysts, while olefin coordination polymerization catalysts include metallocene catalysts, high-efficiency Ziegler Natta (Z-N) catalysts, and non metallocene catalysts.
Five kinds of new-type non-metallocene catalyst were synthesized using phenol and its derivatives as raw materials. Ultra-high molecular weight poly(1-octene) drag reducer was obtained by these catalysts from 1-octene through the low temperature liquid phase bulk polymerization process at atmospheric pressure,and the effects of polymerization conditions were investigated. The structures of the catalysts and the properties of poly(1-octene) were characterized by NMR,XPS,DSC,FTIR and XRD.
The experimental results indicate that the stability of the active center of the catalyst can be improved by the phenoxide group containing strong electron-withdrawing atom F,so Cat.4 containing 2,6-difluorophenoxy was chosen as the catalyst for 1-octene polymerization. The optimum conditions for 1-octene polymerization by Cat.4/methylaluminoxane are as follows:0 ℃ and 24 h at the 1st stage,5 ℃ and 144 h at the 2nd stage,n(Al)∶n(Ti)=50∶1,n(1-octene)∶n(Ti)=2 000∶1. Under these conditions,the monomer conversion is 96.9%,Mη of the produced poly(1-octene) is 3.55×106. The product has an amorphous structure and is easily soluble in oil,and its drag reduction rate is 46.9%,which is equivalent to the imported products.
1. Experimental Part
1.1 Main reagents
l TiCl4 (purity 99% (w)), n-butyl lithium (2.4 mol/L hexane solution): Shanghai Aladdin Biochemical Technology Co., Ltd;
l 2,4,6-trimethylphenol, p-methylphenol, p-fluorophenol,2,4,6-trifluorophenol: analytically pure, Sigma Aldrich (Shanghai) Trading Co., Ltd;
l Dimethyl sulfoxide (DMSO): analytically pure, Cambridge Isotope Laboratories, Inc;
l Deuterotoluene: Analytical pure, Beijing Innokai Technology Co., Ltd;
l N-hexane, toluene, 1-octene: analytically pure, Shanghai Aladdin Biochemical Technology Co., Ltd;
l Sodium metal reflux for 48 hours before use;
l Methylaluminoxane (MAO): 10% (w) toluene solution, produced by China National Pharmaceutical Group Chemical Reagent Co., Ltd.
1.2 Characterization Method
l The NMR analysis was carried out using the Bruker AV400 nuclear magnetic resonance spectrometer, with a temperature of 40 ℃ and a solvent of deuterated DMSO.
l The ESCALAB 250 X-ray photoelectron spectrometer from THERMO VG company was used to determine the content of various elements in the catalyst.
l DSC analysis was conducted using TA company's DTG-Q50 differential scanning calorimeter, with a temperature range of -80~200 ℃, a heating rate of 10 ℃/min, and a cooling rate of 20 ℃/min. The second heating curve was taken.
l WAXD analysis was using Bruker D2Phaser X-ray diffractometer, Cu K α Ray, wavelength 1.540 5 × 10-10 m, scanning range 5 °~50 °.
l The intrinsic viscosity was measured using the VISCO 370 viscosity tester from Julabo company, with toluene as the solvent and a temperature of 30 ℃. The viscosity average molecular weight (M) of the product was calculated based on the intrinsic viscosity (Mη).
l According to the method specified in SY/T 6578-2016, a self-designed indoor testing loop is used to conduct drag reduction performance testing.
1.3 Catalyst synthesis and characterization
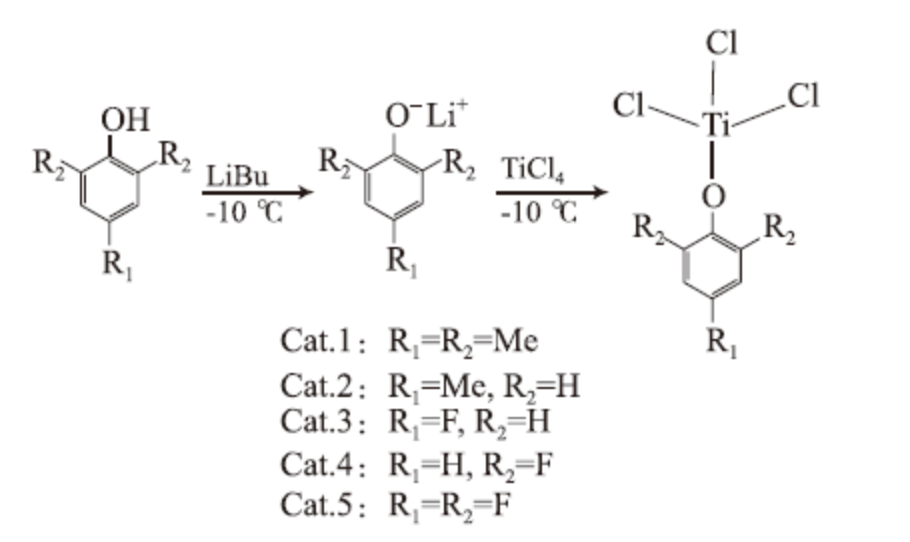
Catalysts Cat.1~Cat.5 were synthesized using phenol and its derivatives, TiCl4 as the main raw materials. The synthesis route of the catalyst is shown in equation (1).
v The synthesis method is as follows: Take a 250 mL Schlenk reaction flask, replace it with nitrogen 4-6 times, and bake it with a hot air gun for 15 minutes;
v After cooling to room temperature, add 1.37 g (10.08 mmol) of 2,4,6-trimethylphenol and 40 mL of toluene in a nitrogen atmosphere;
v Place the reaction bottle in an ice salt bath at -10 ℃, add 4.2 mL of n-butyl lithium (2.4 mol/L hexane solution) dropwise, and react for 1 hour at room temperature for 3 hours to obtain a slightly yellow toluene solution of p-methylphenol lithium compound;
v Then place the reaction bottle in an ice salt bath (-10 ℃), add 1.1 mL of TiCl4 dropwise with a syringe, react for 1 hour, and then heat to 50 ℃ for 5 hours.
v Place the reaction bottle in the refrigerator and let it crystallize.
v Room temperature filtration, vacuum extraction of residual solvents, nitrogen protection, obtained a bright yellow solid product C9H11OTiCl3 (Cat. 1) 2.79 g, with a yield of 96.5%.
The XPS elemental analysis of Cat. 1 (C9H11OTiCl3, molecular weight 289.3) is as follows:C fund 38.1 cal.37.3, Ti fund 15.8 cal.16.5, Cl fund 35.7 cal.36.8; 1H NMR (400 MHz,DMSO);
Chemical shift(δ):2.18(CH3,s,9H), 6.79(CH,s,2H); 13C NMR (125 MHz,DMSO)δ为:154.3(O—C), 131.8(C—C), 130.5(Ar—C), 126.8(Ar—C),21.3(Me),15.8(Me); m/z=289.3
Using the same method, the phenol derivatives were modified to synthesize Cat.2~Cat.5, respectively. The characterization results of each catalyst are as follows.
Light yellow solid Cat.2 (C7H7OTiCl3, molecular weight 261.3), with a yield of 97.3%; XPS element analysis:C fund 33.0 cal.32.1, Ti fund 16.8 cal.18.2, Cl fund 38.7 cal.40.8; 1H NMR(400 MHz,DMSO)δ: 2.19(CH3,s,3H), 6.90(CH,d,2H), 6.79(CH,d,2H);13C NMR(125 MHz,DMSO)δ:155.7(O—C), 132.1(C—C), 129.4(Ar—C), 115.9(Ar—C), 20.6(Me);m/z=261.3
Yellow solid Cat.3 (C6H4FOTiCl3, molecular weight 265.3), with a yield of 95.3%; XPS element analysis:C fund 28.2 cal.27.1,F fund 6.9 cal.7.1,Ti fund 16.9 cal.18.0,Cl fund 38.8 cal.40.1;1H NMR(400 MHz,DMSO)δ:6.97(CH,d,2H),6.79(CH,d,2H);13C NMR(125 MHz,DMSO)δ:156.1(F—C),153.1(O—C),118.1(Ar—C),115.8(Ar—C);m/z=265.3
Brown solid Cat.4 (C6H3F2OTiCl3, molecular weight 283.3), yield 97.0%; XPS element analysis:C fund 26.2 cal.25.4,F fund 12.8 cal.13.4,Ti fund 16.1 cal.16.8,Cl fund 36.8 cal.37.6;1H NMR(400 MHz,DMSO)δ:6.99(CH,d,2H),6.77(CH,d,H);13C NMR(125 MHz,DMSO)δ:156.4(F—C),135.9(O—C),122.8(Ar—C),110.8(Ar—C);m/z=283.3
Brown black solid Cat.5 (C6H2F3OTiCl3, molecular weight 301.3), yield 95.2%; XPS element analysis:C fund 24.6 cal.23.9,F fund 18.5 cal.18.9,Ti fund 15.3 cal.15.9,Cl fund 34.2 cal.35.3;1H NMR(400 MHz,DMSO)δ:7.11(CH,s,H);13C NMR(125 MHz,DMSO)δ:157.3(F—C),156.9(F—C),130.7(O—C),99.8(Ar—C);m/z=301.3
1.4 Synthesis of Drag Reducing Agents
Synthesis of drag reducing agents by using low-temperature and atmospheric pressure liquid phase bulk method
v Take one 250 mL Schlenk reaction flask, replace with nitrogen 4-6 times, bake with a hot air gun for 15 minutes, and fill with nitrogen after cooling.
v Take one glass connector and connect it to a dry ampoule bottle. Replace it with nitrogen 4-5 times and bake it with a hot air gun for 15 minutes. After cooling, weigh a certain amount of catalyst and add it to the ampoule bottle in a nitrogen atmosphere. Add the catalyst to the Schlenk reaction bottle in a nitrogen atmosphere.
v Add MAO to the Schlenk reaction flask using a syringe and alkylate at room temperature for 30 minutes. Add 1-octene monomer at a feeding ratio of n (1-octene) to n (Ti)=2 000:1.
The first stage polymerizes at 0 ℃ for 24 hours, and the second stage reacts at 5 ℃ for 144 hours. Afterwards, the catalyst system was deactivated and filtered using isopropanol. The resulting polymer was dried to constant weight in a vacuum oven at 50 ℃, and the monomer conversion rate and product yield were calculated by weighing.
