Synthesis and mechanism of fluid loss additive for high temperature resistant drilling fluid (Part 1)
Abstract
A water-based fluid loss additive with temperature resistance up to 220 ℃ was prepared by using acrylamide(AM),allyl polyethylene ether(APEG-1000),sodium p-styrenesulfonate(SSS),and octadecyl dimethyl allyl ammonium chloride(DMAAC-18) as polymerization monomers and azodiisobutymidine hydrochloride(V50) as initiator. The prepared fluid loss additive was characterized by FTIR,TG,SEM,EDS and AFM.
The optimal synthesis conditions obtained by single factor optimization method are as follows:n(AM):n(APEG-1000):n(SSS):n(DMAAC-18) = 110:3:10:1,V50 initiator amount 0.4%(w),reaction temperature 60 ℃,monomer content 30%(w),reaction time 4 h,and reaction pH=8. The experimental results show that in the fresh water-based slurry,when the amount of fluid loss additive is 1.25%(w),the medium pressure fluid loss is 7.3 mL,and is 13.2 mL after aging at 220 ℃. When the prepared fluid loss additive is mixed with 5%(w) SMP filtrate reducer and used according to the formula of fresh water base slurry+1.25%(w)prepared fluid loss additive+5%(w)SMP filtrate reducer+0.5%(w)FA367 coating agent,the high-temperature and high-pressure fluid loss aging at 220 ℃ is only 13.6 mL.
The large oil and gas reservoirs discovered in recent years are mainly distributed in deep and ultra deep layers, and the environmental temperature may rise to 200℃ or higher, which poses a challenge to drilling fluids under high temperature and pressure. As an important additive in drilling fluids, fluid loss agents play a crucial role in safe, rapid, and efficient drilling. The adsorption group of the fluid loss agent is adsorbed on the clay particles, while the hydration group forms a hydration film on the surface of the clay, keeping the clay particles evenly dispersed and blocking the pore gaps. Therefore, drilling fluid can form low permeability and dense filter cakes on the wellbore, thereby stabilizing the wellbore and reducing filtration. A fluid loss agent can be synthesized by selecting monomers with strong adsorption and temperature resistant groups, enhancing the thermal stability of the molecular main chain, and monomers that inhibit desorption at high temperatures.
Acrylamide (AM), as the main monomer of polymer molecules, has strong hydration and adsorption abilities of amide groups on the side chains; Allyl polyoxyethylene ether (APEG-1000) is adsorbed on the filter cake through hydrogen bonding; Sodium styrene sulfonate (SSS) enhances the thermal stability of the filtrate reducer due to its sulfonic acid group and steric hindrance effect in the benzene ring, making it suitable for use in some deep wells; Octadecyl dimethyl allyl ammonium chloride (DMAAC-18) has a positively charged nitrogen ion hydrophobic long chain, which can improve the temperature resistance of fluid loss agents.
This article selects four monomers, AM, APEG-1000, SSS, and DMAAC-18, as structural monomers, to synthesize a high-temperature resistant water-based drilling fluid loss reducer. The filtration performance, rheological performance, thermal performance, and adsorption mechanism of the reducer were studied.
1. Experimental Part
1.1 Reagents and Instruments
l AM, SSS, NaOH, anhydrous Na2CO3, azodiisobutymidine hydrochloride (V50): analytical pure, Chengdu Cologne Chemical Reagent Factory;
l APEG-1000 (1000 g/mol): Industrial product, Jiangsu Hai'an Petrochemical Plant;
l DMAAC-18: Industrial Products, Jiangsu Fumiao Chemical Reagent Factory.
l Six speed rotary viscometer (ZNN-D6 type), mud water loss tester (ZNS-2 type), high-temperature roller heating furnace (BRGL-7 type), high-temperature high-pressure water loss tester (GGS71-A type): Qingdao Tongchun Petroleum Instrument Co., Ltd;
l Fourier transform infrared spectrometer (WQF520 type): Beijing Ruili Analytical Instrument Co., Ltd;
l TG-DTG synchronous analyzer (STA-449F3 type): NETZSCH (Shanghai) Machinery Instrument Co., Ltd;
l Scanning electron microscope (ZEISS EVO MA15 type): Carl Zeiss Microscopic Imaging Co., Ltd., Germany;
l Atomic Force Microscope (Dimension Icon type): Bruker, Germany.
1.2 Synthesis of Fluid Loss Additives
Using the aqueous solution polymerization method, a certain amount of AM, APEG-1000, SSS, and DMAAC-18 were sequentially weighed and placed in a three necked flask. Deionized water was added and stirred to dissolve the mixture. The pH was adjusted with 2 mol/L NaOH solution, and nitrogen gas was added to the solution for 10 minutes. The water bath temperature was set. When the solution system reached the reaction temperature, initiator V50 was added and stirred to dissolve the initiator. The polymerization reaction began, and after a period of reaction, a gelatinous polymer was obtained. After washing multiple times in anhydrous ethanol, drying in a vacuum oven, and crushing to obtain a white powder like fluid loss reducer.
1.3 Structural Characterization of Fluid Loss Additives
v Using KBr mixed compression, FTIR characterization of the sample was carried out;
v Using thermal analysis method, the TG curve of the sample was obtained under nitrogen protection, a heating rate of 10 ℃/min, and a temperature range of 40-600 ℃.
1.4 Preparation of drilling fluid base slurry
Measure a certain amount of tap water, add 4% bentonite by mass of tap water, and then add 4% anhydrous Na2CO3 by mass of bentonite. Mix with a constant speed mixer at a speed of 300 r/min for 2 hours, and let it stand for 24 hours to obtain a fresh water base slurry.
1.5 Rheological and filtration properties of drilling fluid
Determine the rheological properties and fluid loss reduction of drilling fluid according to the method specified in GB/T 16783.1-2014.
Add a certain amount of filtrate reducer to the freshwater base slurry, stir at high speed to dissolve, and obtain the filtrate reducer base slurry.
Take a certain amount of prepared filtrate reducer base slurry and the filtrate reducer base slurry aged for 16 hours using a roller heating furnace. After high-speed stirring, measure the rheological parameters using a six speed rotary viscometer, measure the medium pressure (API) filtration rate using a slurry water loss tester, and measure the high temperature and high pressure (HTHP) filtration rate using a high-temperature and high-pressure water loss tester.
2. Results and Discussion
2.1 Optimization of synthesis conditions for high-temperature resistant drilling fluid loss agent
2.1.1 Optimization of monomer ratio
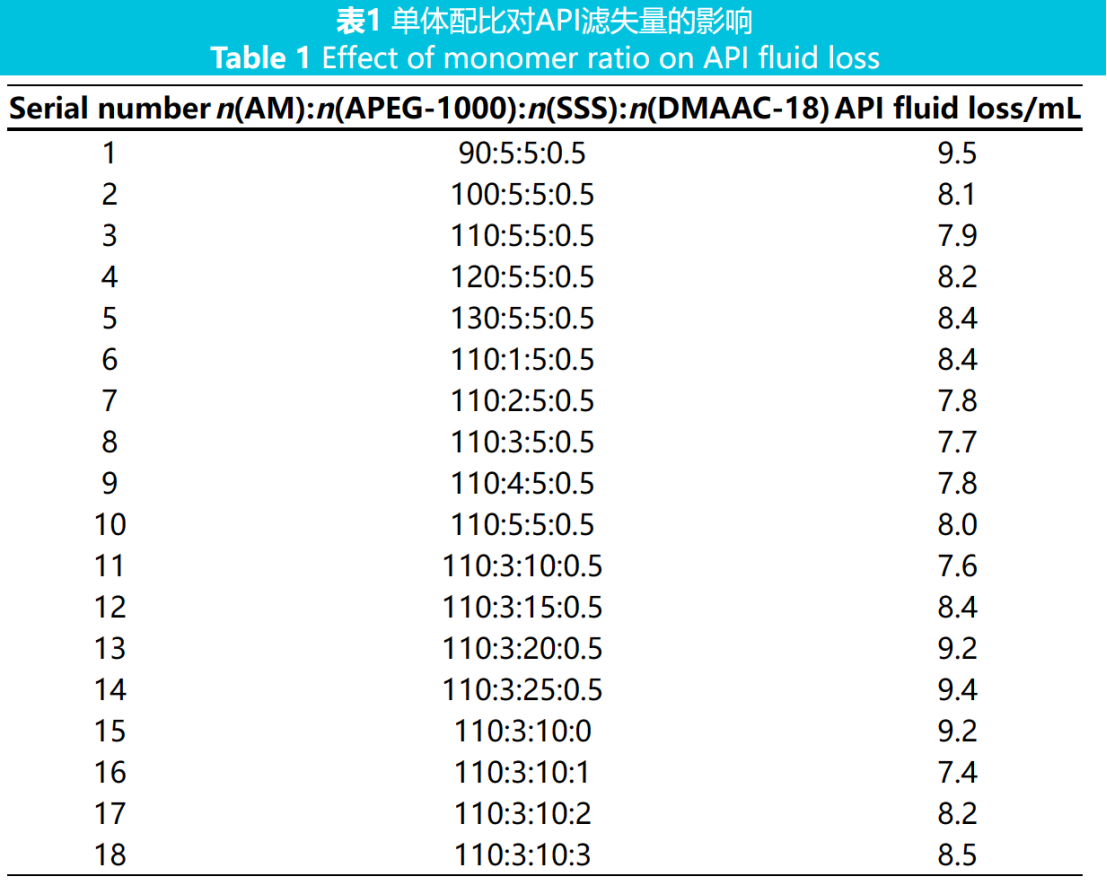
The ratio of reactive monomers has a significant impact on the content of functional groups in the synthesized polymer and the synergistic effect between functional groups. The constant total monomer content is 30% (w, percentage by mass of the solution, the same below), the initiator dosage is 0.4% (w, percentage by mass of the monomer, the same below), the reaction temperature is 60℃, the reaction time is 4 hours, the reaction pH is 7, and a series of water loss reducing agent products are synthesized by changing the monomer ratio. Add 1.25% (w, mass fraction of freshwater based slurry, the same below) of a water loss reducer to the freshwater based slurry, and measure the API filtration rate. The results are shown in Table 1.
From Table 1, it can be seen that with the change of monomer ratio, the filtration performance of the fluid loss agent also changes; When the monomer ratio is n(AM):n(APEG-1000):n(SSS):n(DMAAC-18)=110:3:10:1, the API filtration rate is 7.4 mL, and the best filtration capacity is achieved. This indicates that under the monomer ratio, the prepared fluid loss agent contains appropriate functional group ratios, and there is a good synergistic effect between the molecular functional groups of the fluid loss agent, which can effectively reduce the filtration loss.
Conditions:monomer total content 30%(w),V50 initiator amount 0.4%(w),60 ℃,4 h,pH=7.
AM:acrylamide;APEG-1000:allyl polyethylene ether;SSS:sodium p-styrene sulfonate;DMAAE-18:octadecyl dimethyl allyl ammonium chloride;API:medium pressure;V50:azodiisobutymidine hydrochloride.
2.1.2 Optimization of initiator dosage
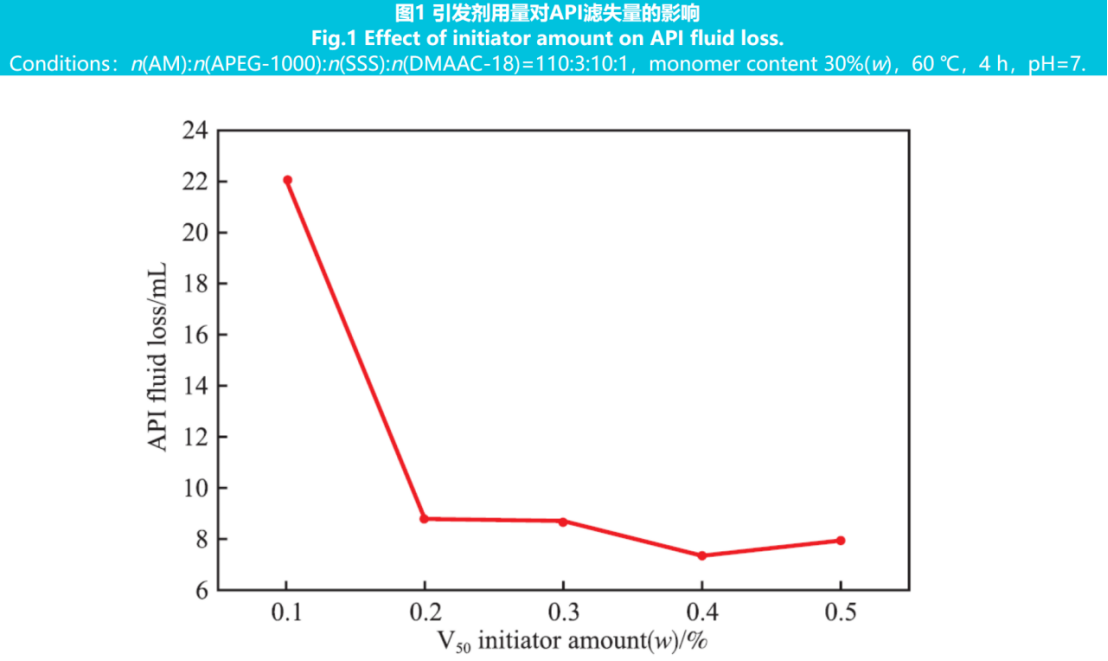
The amount of initiator affects the reaction rate and polymer molecular weight by controlling the number of free radicals, thereby affecting the polymer's filtration reduction effect. A constant monomer ratio of n(AM):n(APEG-1000):n(SSS):n(DMAAC-18)=110:3:10:1, with a total monomer content of 30% (w), a reaction temperature of 60℃, a reaction time of 4 hours, and a reaction pH of 7. A series of fluid loss agent products were synthesized by changing the amount of initiator. Add 1.25% (w) of fluid loss agent to the freshwater base slurry and measure the API filtration rate. The results are shown in Figure 1.
From Figure 1, it can be seen that when the dosage of initiator is 0.4% (w), the best filtration reduction effect is achieved, and the API filtration rate reaches a minimum of 7.4 mL. This is because when the amount of initiator is too low, the free radical generated by decomposition is too small, resulting in low initiation efficiency and low polymer molecular weight and poor filtration failure; When the amount of initiator is too high, the decomposed free radicals are too many, the reaction activity is high, and the mutual termination rate of free radicals also increases, leading to faster polymerization rate, early termination of the reaction, and low polymer molecular weight. Therefore, the initiator dosage was determined to be 0.4% (w).
2.1.3 Optimization of reaction temperature
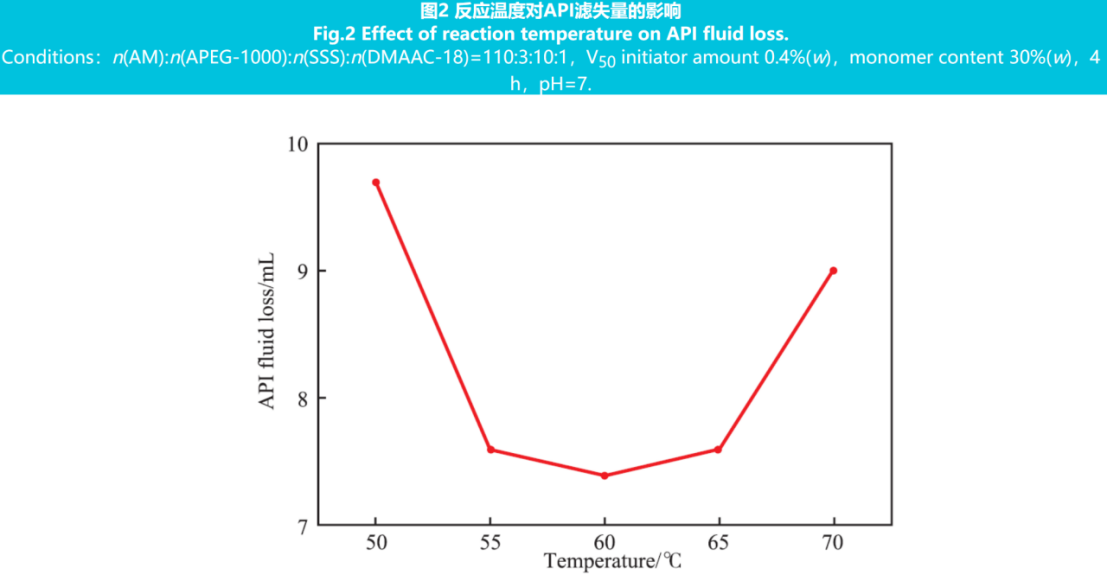
The reaction temperature controls the reaction rate and polymer molecular weight by influencing factors such as initiator thermal decomposition and monomer activity. Constant monomer ratio n(AM):n(APEG-1000):n(SSS):n(DMAAC-18)=110:3:10:1, initiator dosage 0.4% (w), total monomer content 30% (w), reaction time 4 hours, reaction pH=7, and changing reaction temperature to synthesize a series of fluid loss agent products. Add 1.25% (w) of water loss reducing agent to the freshwater base slurry and measure the API filtration rate. The results are shown in Figure 2.
From Figure 2, it can be seen that when the reaction temperature is 60 ℃, the API filtration rate is 7.4 mL, and the best filtration reduction effect is achieved.
This is because when the reaction temperature is low, the decomposition rate of the initiator is slow, the content of free radicals generated is low, and the chain growth rate slows down, which prevents the monomer from fully reacting and reduces the molecular weight of the polymer, affecting the performance of the fluid loss agent. When the reaction temperature is high, the decomposition rate of the initiator accelerates, producing a large number of active free radicals, resulting in explosive polymerization, early termination of the reaction, and lower molecular weight of the polymer. Therefore, the reaction temperature was determined to be 60℃.