Synthesis and Properties of Acrylamide/Methyl Acryloyl Oxygen Ethyl Dimethyl Ammonium Propyl Sulfonic Acid Copolymer
Abstract
Dimethylaminoethyl acrylate (DMAEA) was prepared using dimethylaminoethanol and acryloyl chloride as raw materials;DMAEA reacts with 1,3-propanesulfolactone to produce betaine type vinyl zwitterionic monomer - methacryloxyethyl dimethylpropanesulfonic acid ammonium (MSBM);MSBM co polymerized with 50% acrylamide aqueous solution to synthesize amphoteric polymer acrylamide/methacryloyloxyethyl dimethylpropanesulfonate ammonium (AM/MSBM), and its structure was confirmed by 1H NMR and IR. The effects of mineralization, shear action, and temperature on the viscosity of AM/MSBM were studied. The results indicate that AM/MSBM exhibits anti electrolyte solution behavior. Under experimental conditions (salinity of 1075.9mg·L-1, temperature of 45-75℃, shear rate of 2000rpm), the viscosity retention rate of AM/MSBM is over 90%. The simulation oil displacement experiment results show that AM/MSBM can improve oil recovery by 13.2%.
Acrylamide polymers are a collective term for homopolymers of acrylamide (AM) and copolymers obtained by polymerization of AM with other monomers. Most of these polymers are linear water-soluble polymers and are one of the most widely used water-soluble polymer products in oil fields. The most representative one is the copolymer of AM and acrylic acid.
As oil field development enters the later stage, improving oil recovery technology has become one of the important measures for sustainable development of oil fields. The production, performance, and application research of polyacrylamide (PAM) are receiving increasing attention. PAM and its derivatives are widely used in tertiary oil recovery in the petroleum industry, becoming an important means to improve crude oil recovery.
PAM was successfully developed in 1893, but it was not until 1954 that it was industrially produced. China began producing PAM in the early 1960s, with a relatively small production scale. Ding Wei et al. synthesized 3- (acrylamide propyl dimethylamine) propanesulfonate (DMAPAAS) from acrylamide propyl dimethylamine (DMAPAA) and 1,3-propylsulfolactone at 55℃ for 20 hours;DMAPAAS and AM underwent free radical copolymerization reaction in salt solution to synthesize a zero net charge sulfonated betaine type zwitterionic copolymer P (AM-DMAPAAS).
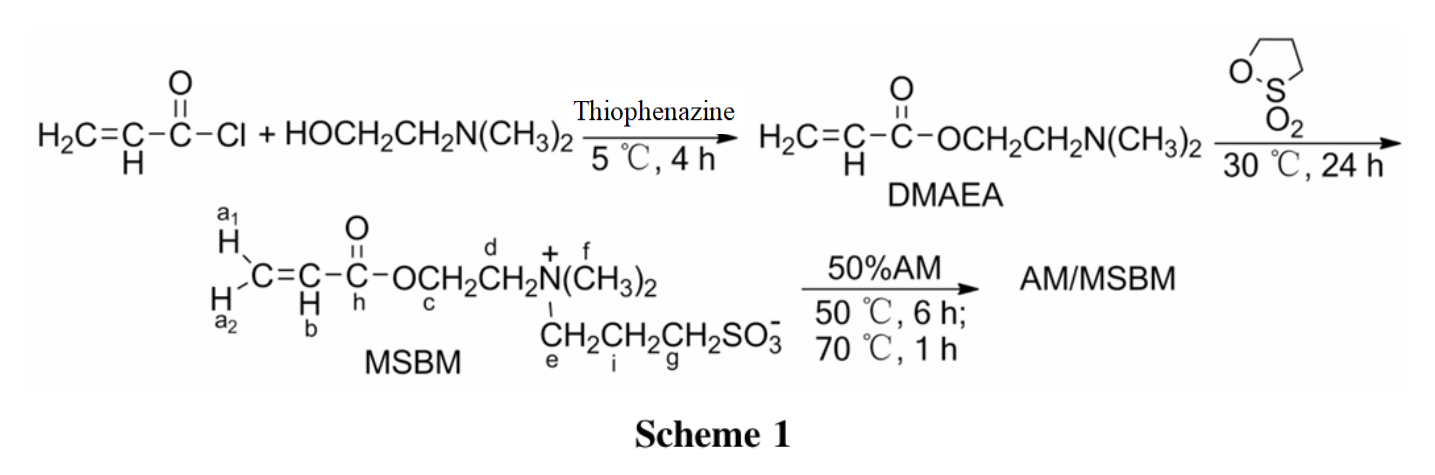
On the basis of literature methods, this article uses dimethylaminoethanol and acryloyl chloride as raw materials to prepare dimethylaminoethyl acrylate (DMAEA);DMAEA reacts with 1,3-propanesulfolactone to produce betaine type vinyl zwitterionic monomer - methacryloxyethyl dimethylpropanesulfonic acid ammonium (MSBM);MSBM co polymerized with 50% AM aqueous solution to synthesize amphoteric polymer acrylamide/methacryloyloxyethyl dimethylpropanesulfonate ammonium (AM/MSBM, Scheme 1), and its structure was confirmed by 1H NMR and IR. The effects of mineralization, shear action, and temperature on the viscosity of AM/MSBM were studied.
1. Experimental Section
1.1 Instruments and Reagents
l Bruker-AVANCE DMX500 nuclear magnetic resonance instrument (CD3OD as solvent, TMS as internal standard);
l Bruker-Tensor 27 Fourier Transform Infrared Spectrometer (KBr Tablet);
l 2WAJ Abbe refractometer;
l KL300 emulsifier;
l Diluted Ubbelohde viscometer.
u 1,3-propanesulfolactone, analytically pure, Hubei Yuancheng Saichuang Technology Co., Ltd;
u 50% AM aqueous solution, industrial product, polymer plant of Daqing Refining and Chemical Company;
u Span-80, Tween-80, industrial products, Lushun Chemical Plant;
u Hydrogenated kerosene, industrial product, Daqing Petrochemical Plant;
u All other reagents used are analytical grade.
1.2 Synthesis
(1) Synthesis of MSBM
Acryloyl chloride 1 mol, dimethylaminoethanol 2.05 mol, polymerization inhibitor phenothiazine 3.2g, reacted at 5℃ for 4 hours. Filter, concentrate the filtrate at atmospheric pressure, and then steam it under reduced pressure to remove residual unreacted substances. Collect a fraction of 75℃/29.4 KPa to obtain a colorless transparent liquid DMAEA.
Add 10g of DMAEA and 150mL of acetone to a four necked bottle, stir and add 6g of propanesulfone dropwise. React at 30℃ for 24 hours. Filter, vacuum dry filter cake to obtain white solid MSBM 3.9g.
(2) Synthesis of AM/MSBM
Add 10mL of Span-80, 15mL of Tween-80, and 120mL of hydrogenated kerosene to the reaction flask, stir to dissolve them; Add 200mL of 50% AM aqueous solution and 3.9g of MSBM, 150mL of deionized water, an appropriate amount of sodium chloride and 8mL of EDTA, and use an emulsifying mechanism to form a stable emulsion. Transfer to a four necked flask, add nitrogen for deoxygenation, stir and add 3mg of AIBN at 50℃ for 6 hours, and react at 70℃ for 1 hour. Centrifuge with a 10000r• min-1 centrifuge and vacuum dry the precipitate to obtain a white solid AM/MSBM.
1.3 Oil Displacement Performance Testing
Test conditions: The displacement core is a quartz sand epoxy cemented heterogeneous artificial core (4.5cm×4.5cm×30cm), with a permeability variation coefficient of 0.65. The experimental oil used was degassed crude oil from Daqing Oilfield, simulated saline water (mineralization degree 4303.60mg•L-1), polymer solution 1000mg•L-1, and experimental temperature of 45℃.
Test steps: Evacuate the core for 3.5 hours, measure the porosity of the artificial core with saturated saline water, and dry it at 45℃ for 2 hours. Use degassed crude oil to drive water until no water is produced at the model outlet, and determine the original oil saturation. At a displacement rate of 1m•d-1, water was driven to the outlet of the core model with a water content of >98%, and a polymer solution plug of 0.38PV was injected.
2. Results and Discussion
2.1 Characterization
According to the IR spectrum analysis of AM/MSBM (figure omitted), the characteristic peak at 3418cm-1 is the N-H asymmetric stretching vibration absorption peak, the characteristic peak at 3192cm-1 is the N-H symmetric stretching vibration absorption peak, the characteristic peak at 1483cm-1 is the -CH2- bending vibration absorption peak, the characteristic peak at 1320cm-1 is the C-N stretching vibration absorption peak, the characteristic peak at 2912cm-1 is the C-H stretching vibration absorption peak, the characteristic peak at 1669cm-1 is the amide C=O stretching vibration absorption peak, and the characteristic peak at 1187cm-1 is the sulfonic acid group characteristic absorption peak.
According to the analysis of the 1H NMR spectra of AM/MSBM (figure omitted), it can be seen that the a-H characteristic absorption peak is located at δ 1.87, the b-H characteristic absorption peak is located at δ 2.43, the c-H characteristic absorption is located at δ 1.32, the d-H characteristic absorption is located at δ 3.78, the e-H characteristic absorption is located at δ 3.95, the f-H characteristic absorption is located at δ 3.43, the g-H characteristic absorption is located at δ 2.47, the h-H characteristic absorption is located at δ 3.83, the i-H characteristic absorption is located at δ 2.32, and the j-H characteristic absorption is located at δ 3.18. Based on the comprehensive IR analysis results, it can be concluded that AM/MSBM has been successfully synthesized.
2.2 Performance of AM/MSBM
(1)Performance of Anti Electrolyte Solution
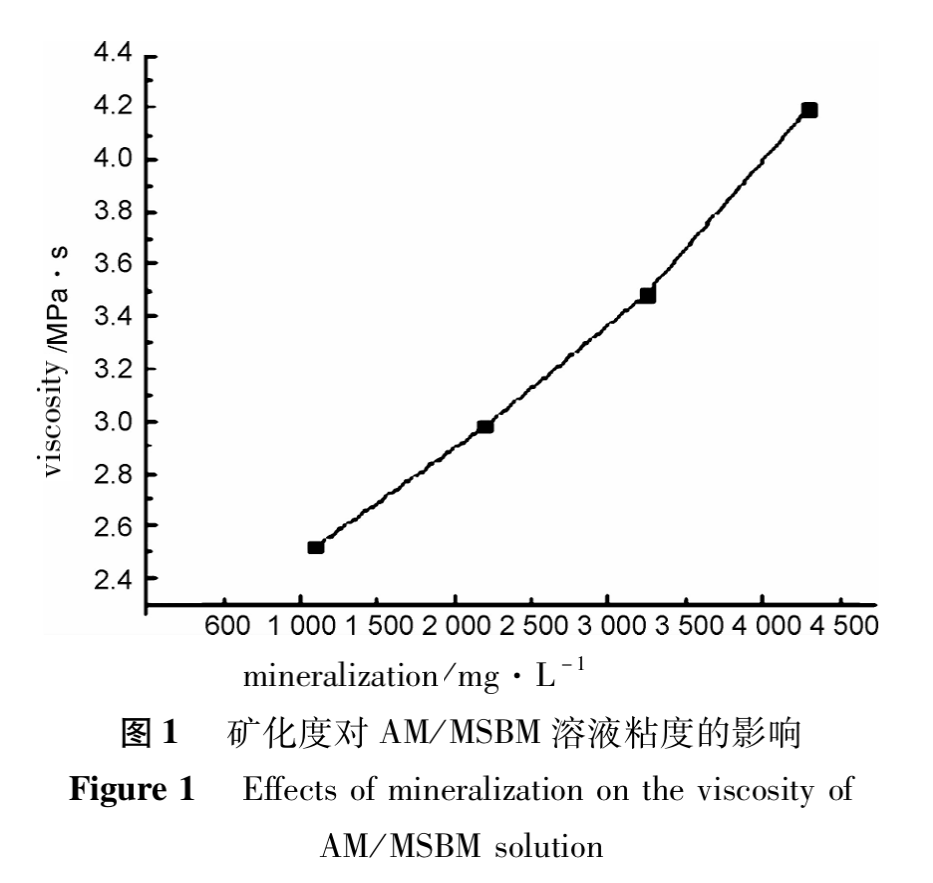
The effect of salinity on the viscosity of AM/MSBM solution was tested at 45℃, shear rate of 7.37s-1, and c (AM/MSBM)=100mg•L-1. The results are shown in Figure 1. As shown in Figure 1, the AM/MSBM solution exhibits anti electrolyte behavior, and its viscosity increases significantly with increasing mineralization degree.
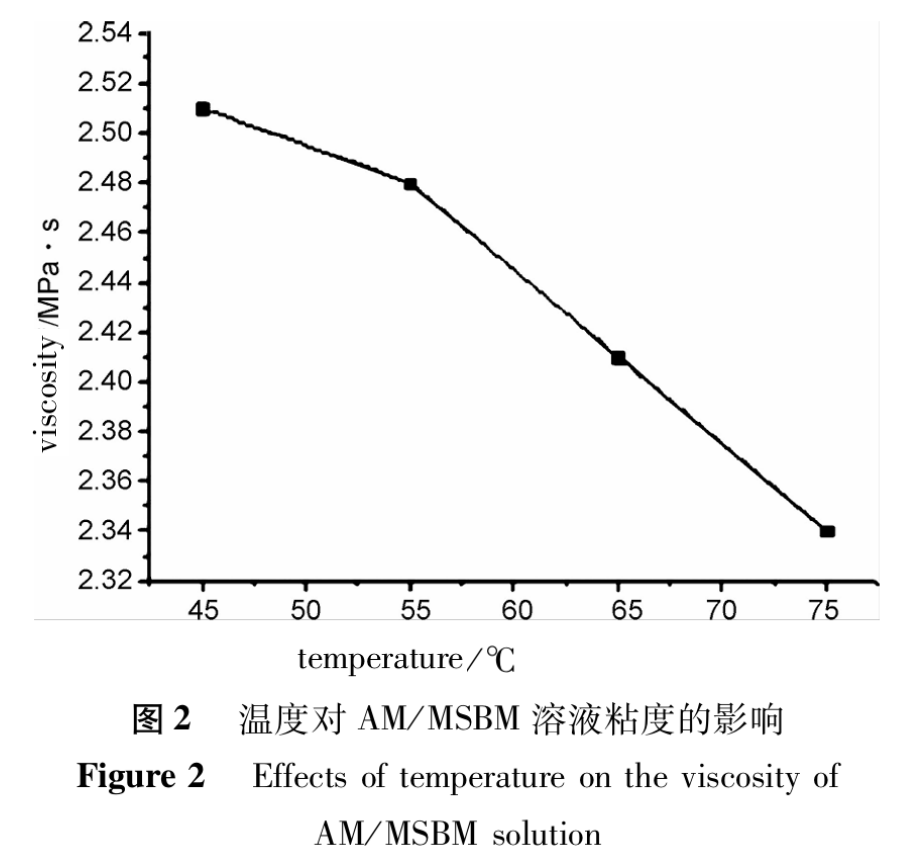
The salinity of saline solution is 1075.9mg•L-1, with a shear rate of 7.37s-1 and c (AM/MSBM)=100mg•L-1. The effect of temperature on the viscosity of AM/MSBM solution was studied, and the results are shown in Figure 2. As shown in Figure 2, the viscosity retention rate of AM/MSBM solution is >90% at 45-75℃.
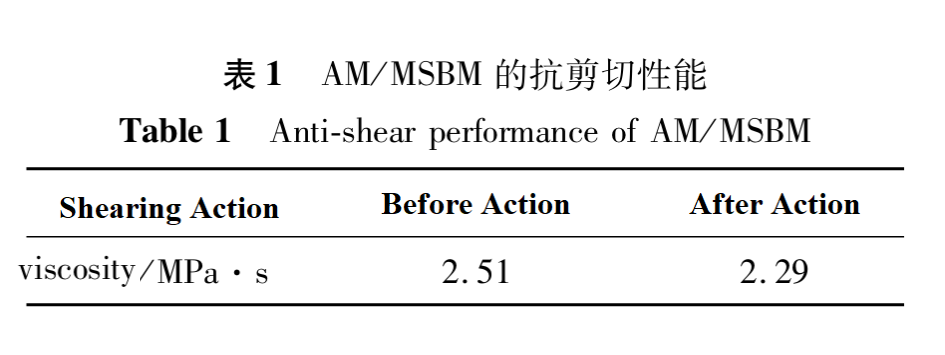
The salinity of saline water was 1075.9mg•L-1, with c (AM/MSBM)=100mg•L-1. The effect of shear on viscosity was studied at 45℃ and 2000rpm for 1 hour. The results are shown in Table 1. From Table 1, it can be seen that AM/MSBM maintains a viscosity retention rate of >90% before and after shear action.
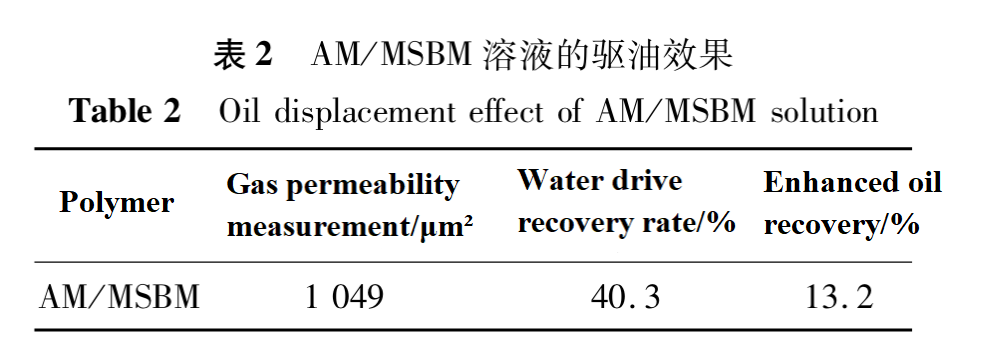
(2) Oil Displacement Performance
Table 2 shows the oil displacement effect of AM/MSBM solution. As shown in Table 2, using AM/MSBM solution for oil recovery can increase the recovery rate by 13.2%.