Study on the Intrinsic Viscosity of Polymer Drag Reducing Agents prepared by Bulk Polymerization Method(Part 2)
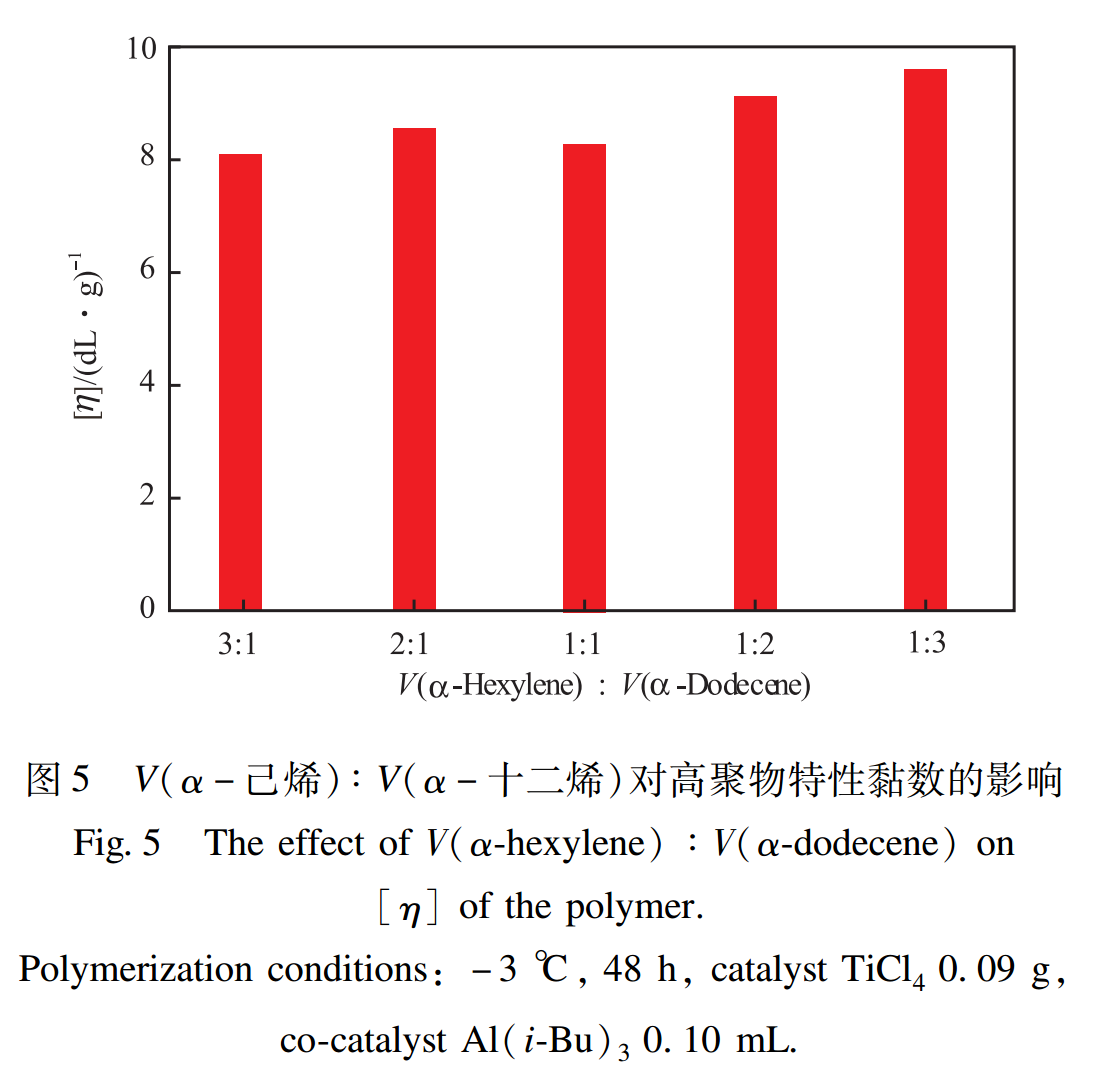
2.4 Effect of V(α-hexylene)/V(α-dodecene) ratio
The effect of V(α-hexylene)/V(α-dodecene) ratio on the intrinsic viscosity of polymers is shown in Figure 5. As shown in Figure 5, the effect of this ratio on the intrinsic viscosity is not very significant. Except for V(α-hexylene): V(α-dodecene) is 1:1, the intrinsic viscosity decreases slightly, but overall, the intrinsic viscosity increases with the increase of α-Dodecene content. Select V(α-hexylene): V(α-dodecene)=1:3 is more suitable.
The single factor experimental results indicate that the optimal synthesis conditions for the polymer are:
main catalyst TiCl4 dosage 0.08g, reaction temperature -5℃, V(α-hexylene): V(α-dodecene)=1:3, co catalyst Al(i-Bu)3 dosage of 0.10mL, reaction time of 48 hours. The intrinsic viscosity of the polymer reached 11.10 dL/g under this condition.
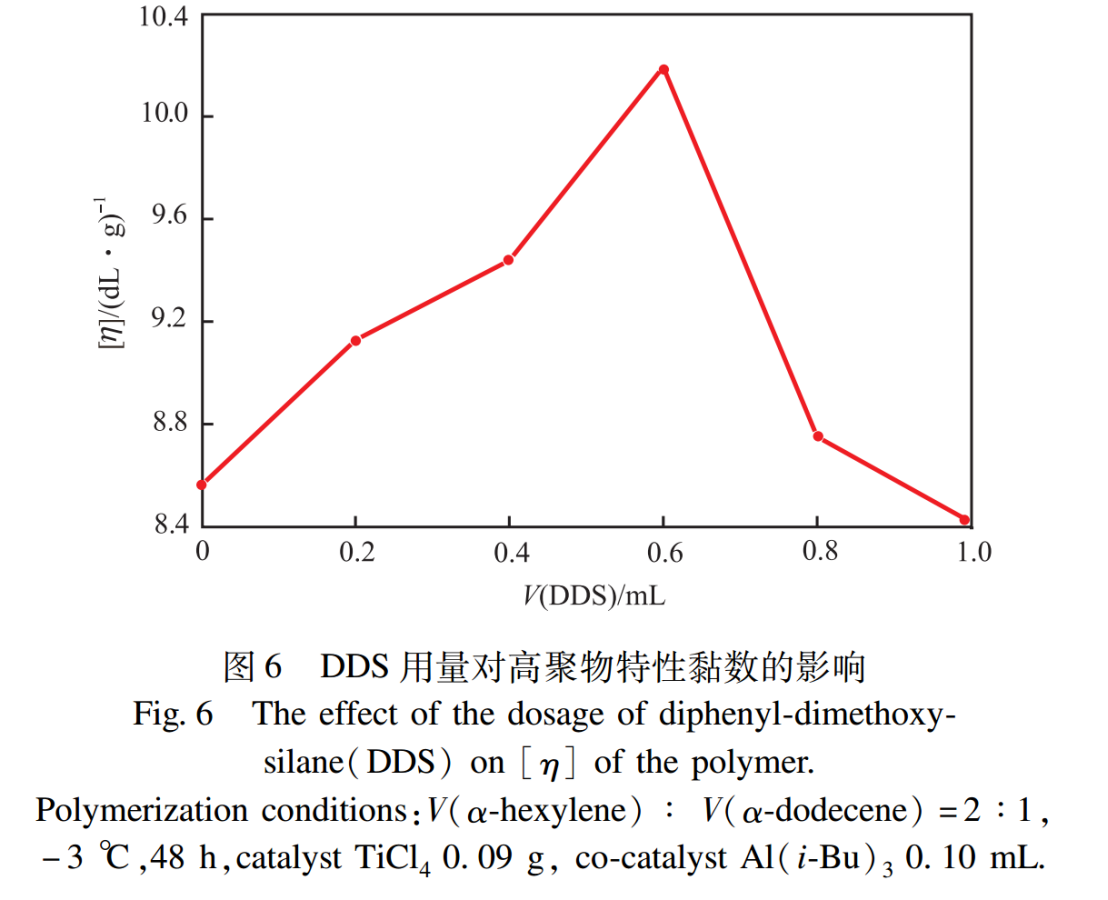
2.5 The Effect of DDS Dosage
In the new efficient Ziegler Natta catalytic system, the addition of electron donors can significantly alter the performance of the catalyst, control the relative molecular weight and its distribution, and affect the viscosity of the polymer. DDS is a commonly used external electron donor that can improve the activity and stereoselectivity of catalytic systems, while also affect the microstructure and relative molecular weight of polymers.
The effect of DDS dosage on the intrinsic viscosity of polymers is shown in Figure 6. From Figure 6, it can be seen that the intrinsic viscosity first increases and then decreases with the increase of DDS dosage, which indicates that the addition of DDS can only improve the intrinsic viscosity within an appropriate range. This is because when the dosage of DDS is low, DDS can promote the conversion of the random active center of Al (i-Bu)3 to the isotactic active center, increase the number of isotactic active centers, and thus increase the intrinsic viscosity of the polymer; But when the dosage of DDS is high, excessive DDS binds to Al (i-Bu)3, resulting in a decrease in the number of Al (i-Bu)3, premature deactivation of active chain segments, and a decrease in intrinsic viscosity.
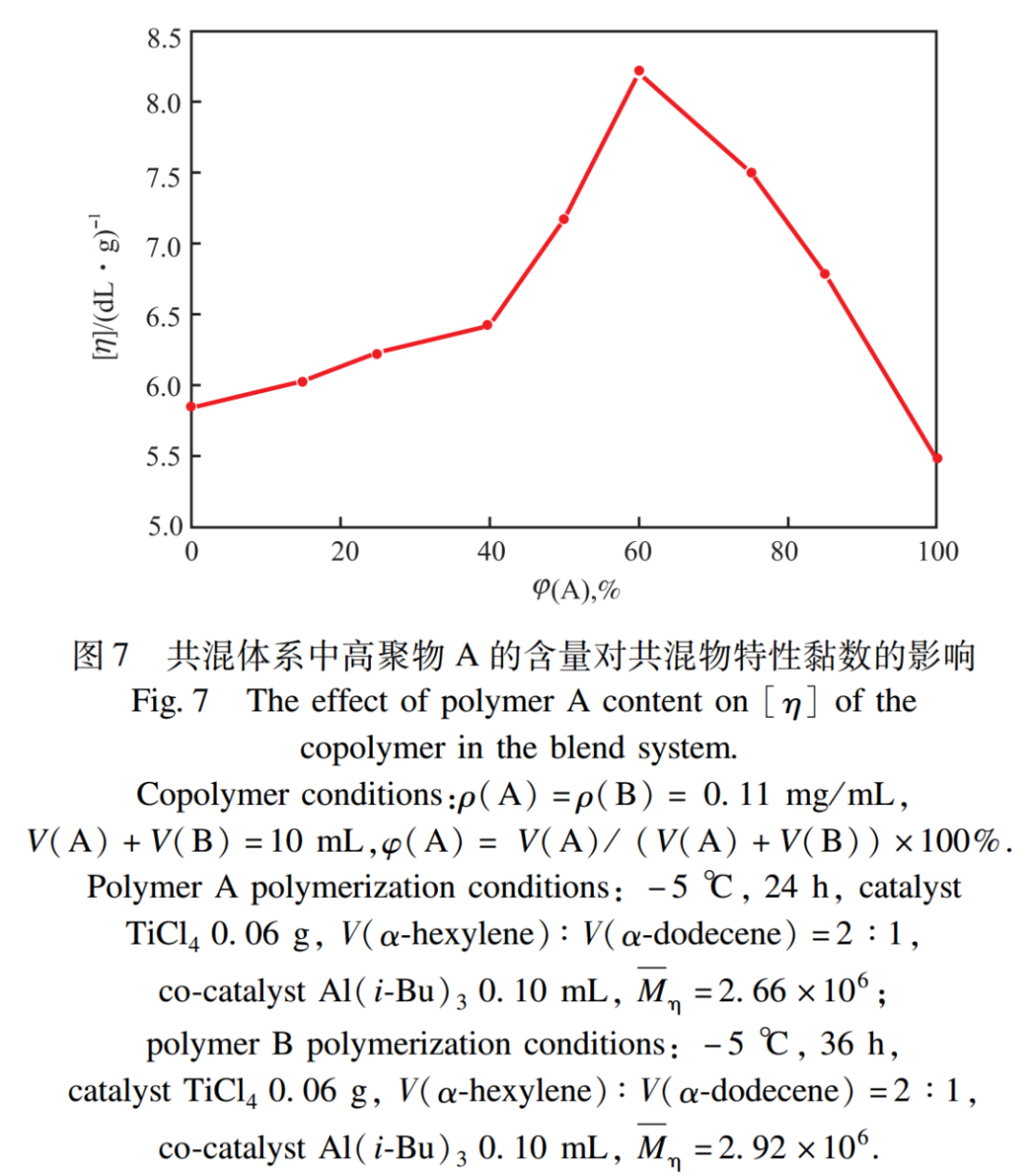
2.6 The Effect of Polymer Blending
The effect of blending A and B polymers on the intrinsic viscosity of the blend is shown in Figure 7. As shown in Figure 7, the intrinsic viscosity of the blend first increases and then decreases with the increase of polymer A content. When the volume fraction of polymer A is 60%, the intrinsic viscosity of the blend reaches its maximum. This is because the system blends to form an interlocking interwoven structure, which increases the flow resistance of the fluid and leads to an increase in the intrinsic viscosity of the blend; The intrinsic viscosity of blends is greater than that of a single polymer.
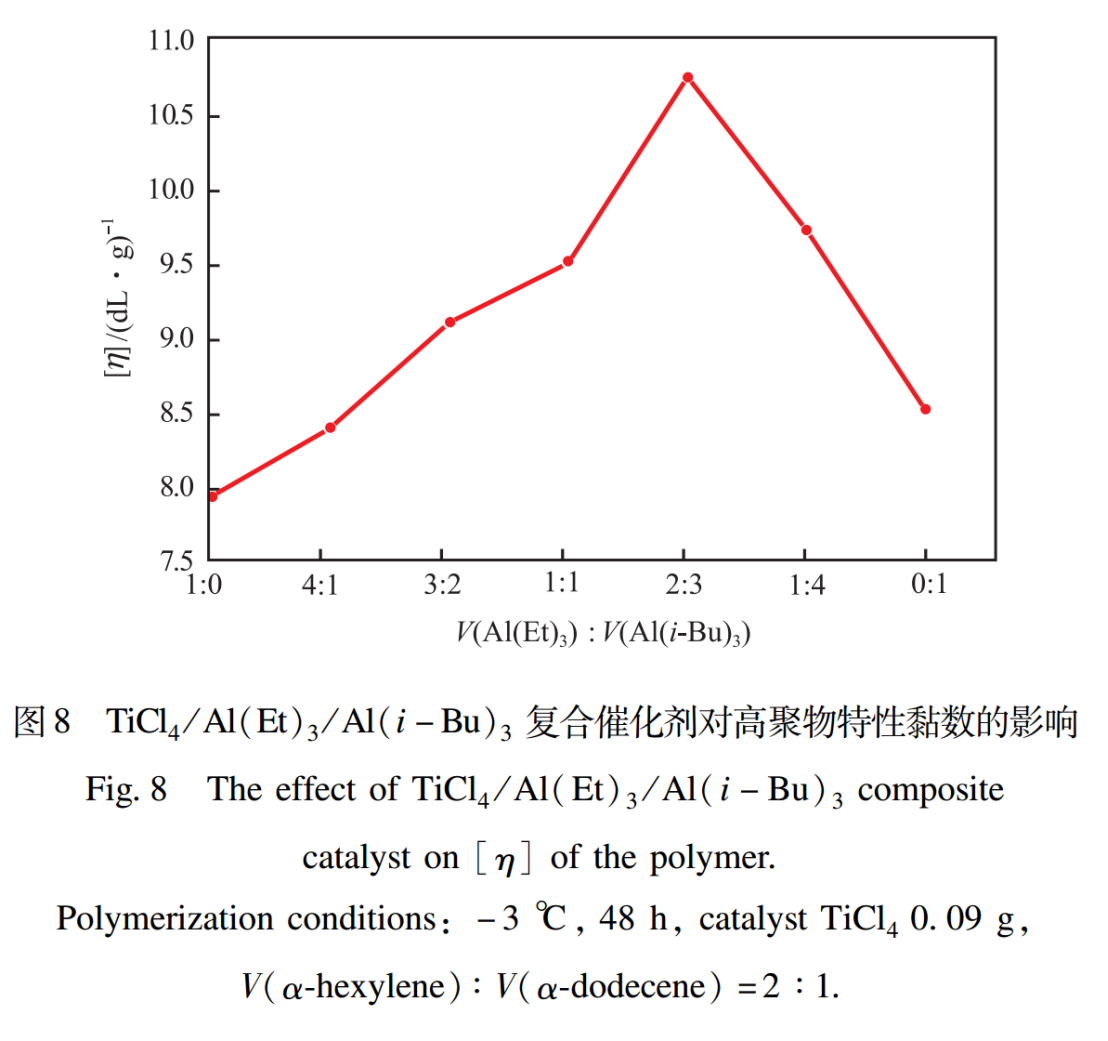
2.7 Effect of Composite Catalysts
The effect of TiCl4/Al (Et) 3/Al (i-Bu) 3 composite catalyst on the intrinsic viscosity of polymers is shown in Figure 8. From Figure 8, it can be seen that the intrinsic viscosity number first increases and then decreases with the increase of Al (i-Bu)3 content. When V (Al (Et)3): V (Al (i-Bu)3)=2:3, the intrinsic viscosity value reaches its maximum. This is because when Al (i-Bu)3 is a cocatalyst, the polymerization reaction rate is fast, while when Al (Et)3 is a cocatalyst, the polymerization reaction rate is slow. The polymerization system with fast reaction rate first appears gel effect, and the polymerization system with slow reaction rate later appears gel effect. The combination of Al (i-Bu)3 and Al (Et)3 as two co catalysts can make the reaction rate of the polymerization system more uniform, slowly releasing the reaction heat, which is beneficial for improving the relative molecular weight and increasing the intrinsic viscosity. Therefore, using TiCl4/Al (Et)3/Al (i-Bu)3 as a composite catalyst can increase the intrinsic viscosity of the polymer.
2.8 The Effect of Ultrasound Degradation
Under the action of ultrasound, a large shear stress is generated in the polymer solution, and the polymer solution exhibits obvious ultrasonic degradation characteristics. The fracture of polymer molecular chains caused by ultrasonic shear can achieve the same shear effect as mechanical fracture, while the intrinsic viscosity of the polymer will change.
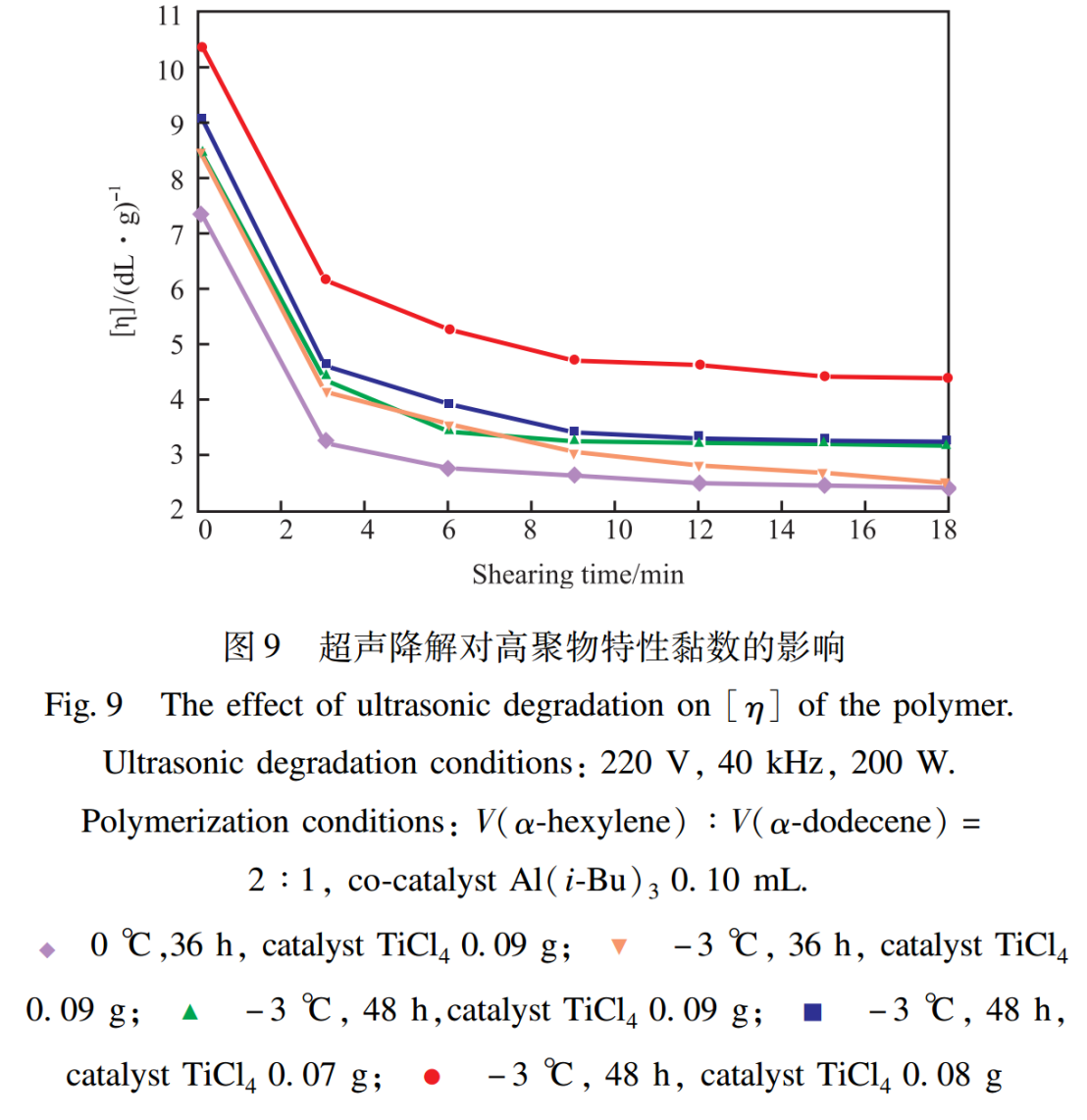
The effect of ultrasonic degradation on the intrinsic viscosity of polymers is shown in Figure 9. From Figure 9, it can be seen that with the extension of shear time, the intrinsic viscosity of polymers shows a decreasing trend, with a decreasing amplitude from fast to slow, and finally tends to stabilize.The larger the initial intrinsic viscosity, the smaller the decrease in intrinsic viscosity, and the greater the remaining intrinsic viscosity after ultrasonic degradation. From this, it can be seen that the larger the initial intrinsic viscosity value (i.e. the larger the average molecular weight of the initial viscosity), the better the shear stability. Therefore, by changing the amount of reactants and reaction conditions, the relative molecular weight of the polymer can be effectively controlled, thereby achieving the goal of optimizing the shear resistance of the polymer. This has important application value.
3. Conclusion
(1) . The XRD and IR characterization results show that the synthesized polymer is a flexible α-Olefin polymer with low crystallinity.
(2) . The optimal synthesis conditions for the polymer are main catalyst TiCl4 dosage 0.08g, reaction temperature -5℃, V(α-hexylene): V(α-dodecene)=1:3, co catalyst Al(i-Bu)3 dosage of 0.10mL, reaction time of 48 hours. The intrinsic viscosity of the polymer reached 11.10 dL/g under this condition.
(3) . The intrinsic viscosity of blends is greater than that of a single polymer; When TiCl4/Al (Et)3/Al (i-Bu)3 is used as a composite catalyst, the intrinsic viscosity of the polymer can be increased; Adding a small amount of DDS can also increase the intrinsic viscosity of the polymer.
(4). After ultrasonic degradation, the intrinsic viscosity of the polymer solution decreases first and then tends to stabilize with the extension of shear time; The larger the initial characteristic viscosity, the smaller the decrease, and the better the shear stability.
