Study on the Intrinsic Viscosity of Polymer Drag Reducing Agents prepared by Bulk Polymerization Method(Part 1)
Abstract
A α-hexylene /α-dodecene copolymer (referred to as polymer) was synthesized by bulk polymerization method with TiCl4/Al(i-Bu)3 as a composite catalyst and α-olefin as monomers. The effects of reaction conditions including catalyst dosage, V(α-hexylene)/V(α-dodecene) ratio,polymer blending and reaction temperature on the intrinsic viscosity of the copolymer were investigated. The experimental results indicate that under optimal conditions for polymer synthesis (main catalyst TiCl4 dosage 0.08g, reaction temperature -5℃, V(α-hexylene): V(α-dodecene)=1:3, co catalyst Al(i-Bu)3 dosage of 0.10mL, reaction time of 48 hours), the intrinsic viscosity of the polymer reached 11.10 dL/g; Adding a small amount of diphenyldimethoxysilane can increase the intrinsic viscosity of the polymer; The intrinsic viscosity of blends is greater than that of a single polymer; When TiCl4/Al (Et)3/Al (i-Bu)3 is used as a composite catalyst, the intrinsic viscosity of the polymer can be increased. The XRD and IR characterization results show that the synthesized polymer is a flexible α-Olefin polymer with low crystallinity.
α-Olefin polymer with high relative molecular weight are an efficient drag reducing agent for crude oil. Adding a small amount of drag reducing agent to the transportation pipeline of crude oil and finished oil can significantly reduce the energy dissipation of the oil in turbulent conditions, increase the transportation volume, increase the flow rate, and improve the safety factor of pipeline operation. At present, the main methods for synthesizing polymer drag reducing agents are solution polymerization and bulk polymerization. Solution polymerization is an early research method for polymerization, which has the drawbacks of low monomer conversion rate and low relative molecular weight of the product. Additionally, the complex solvent post-treatment of the synthesized polymer poses great difficulties for on-site application. Therefore, bulk polymerization is gradually replacing solution polymerization and is considered a more advanced polymerization method at present. Ma Yong pointed out that the magnitude of polymer drag reduction rate can be qualitatively represented by the magnitude of intrinsic viscosity. At the same time, when the polymer undergoes shear degradation, the intrinsic viscosity will also change, so the degree of change in the intrinsic viscosity of the polymer can be used to characterize its shear resistance.
This article adopts the bulk polymerization method to synthesize a α-hexylene /α-dodecene copolymer (referred to as polymer) by using TiCl4/Al(i-Bu)3 as a composite catalyst and α-olefin as monomers; The effects of reaction conditions including catalyst dosage, V(α-hexylene)/V(α-dodecene) ratio,polymer blending and reaction temperature on the intrinsic viscosity of the copolymer were investigated; The structure of the polymer was characterized by XRD and IR methods.
1. Experimental Part
1.1 Reagent
l α-Dodecene: polymer grade, Fluka company, with a water content not exceeding 25mg/L;
l α-Hexylene: purity of 98%, produced by Bailingwei Company, used after dehydration through 5A molecular sieve;
l TiCl4 main catalyst loaded with MgCl2: brown powder, Beijing Institute of Chemical Technology, Sinopec;
l Al (Et) 3: Industrial grade, Burris Druck Reagent Company;
l Al (i-Bu) 3: Analytical pure, Burris Druck Reagent Company;
l Nitrogen: 99.99% purity, Xinjiang Shanxia Electromechanical Equipment Co., Ltd;
l Diphenyldimethoxysilane (DDS): Purity not less than 98%, Xinjiang Urumqi Petrochemical Plant.
1.2 Preparation of High Polymers
The preparation of high polymers was carried out under anhydrous and oxygen free conditions, and all the glass instruments used were dried in a 120℃ blast drying oven for more than 6 hours. The weighing of the main catalyst TiCl4 is carried out in a vacuum glove box, and the auxiliary catalysts Al (Et) 3 or Al (i-Bu) 3 are added under high-purity nitrogen protection.
Before the reaction, vacuum and repeatedly replace the air in the polymerization bottle with high-purity nitrogen gas, and add α-Dodecene, α-Hexylene, co catalyst, main catalyst in sequence under magnetic heating and stirring, and control the required temperature for the reaction. When the system is evenly mixed and the magnetic particles no longer rotate, seal the polymerization bottle and transfer it to a refrigeration room for later reaction. After the reaction is completed, the resulting product is washed with anhydrous ethanol, and then dried to constant weight in a vacuum drying oven (60℃). The dried product is dissolved in n-hexane for the determination of intrinsic viscosity.
1.3 Characterization of Polymers
Using Bruker's Equinox55 infrared spectrometer to characterize the structure of polymers, KBr compression; Characterization of the structure of polymers using the D/MAX-2400 X-ray powder diffraction instrument from Rigaku Corporation, Cu target, Ni filter, wavelength 0.154nm, scanning range 2θ=10~70º.
1.4 Determination of Intrinsic Viscosity
Measure the intrinsic viscosity of the polymer in a glass constant temperature water bath (30℃) with a temperature control accuracy of ±0.01℃. After holding the Ubbelohde viscometer in a glass constant temperature water bath for 10 minutes, the intrinsic viscosity of the polymer is measured, and the flow time is calculated by averaging multiple measurements.
The steps for determining the intrinsic viscosity are as follows: First, use a Ubbelohde viscometer to measure the flow time (t0) of the pure solvent n-hexane; Second, after drying the Ubbelohde viscometer, measure the flow time (t) of the polymer solution. The mass concentration of the measured polymer solution is the same, and the intrinsic viscosity of the polymer is calculated using the following equation (1)([η],dL/g).
.png)
In the equation, ηr is the relative viscosity; ηsp is the increase in specific viscosity; ρ is the mass concentration, g/dL; ηr=t/t0;ηsp=ηr - 1.
2.1 Results and Discussion
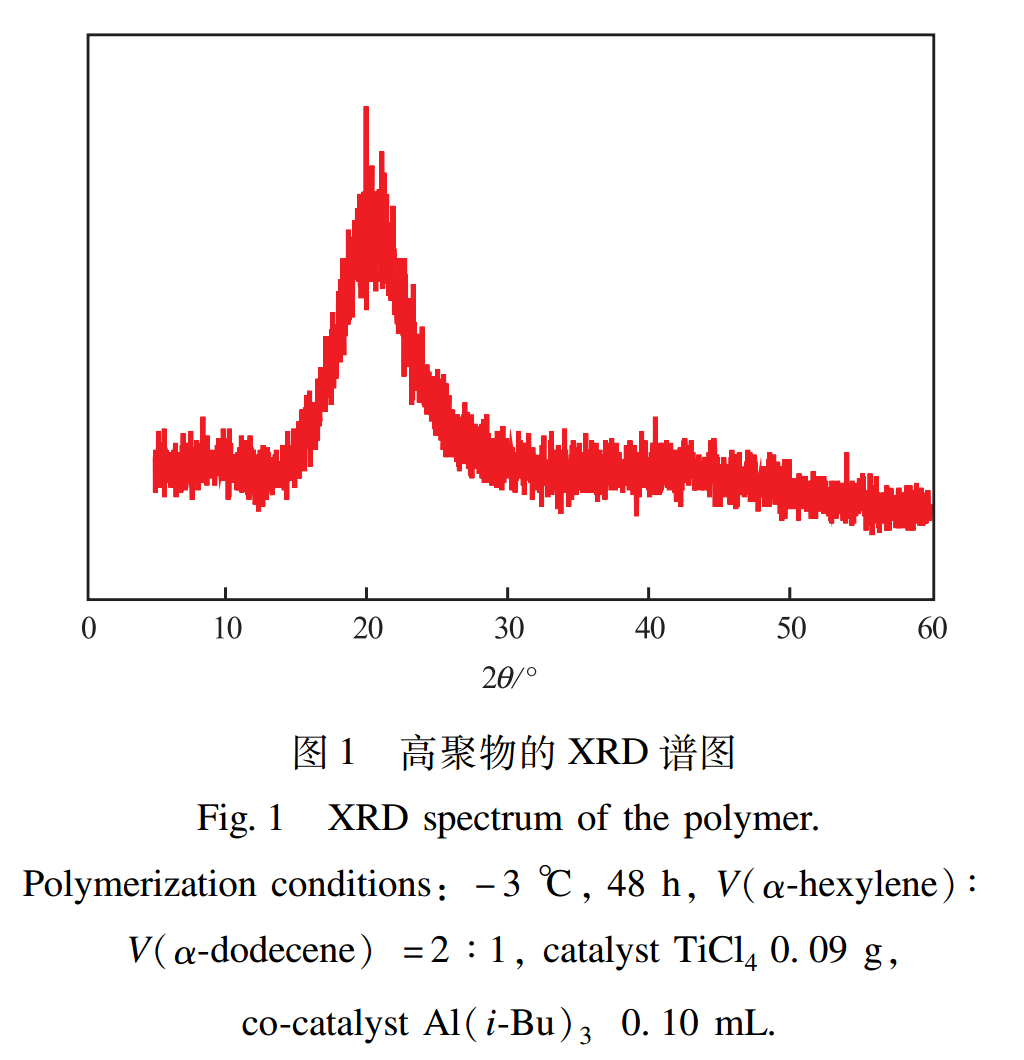
2.1.1 XRD Characterization Results
The XRD spectrum of the polymer is shown in Figure 1. As shown in Figure 1, there is an extremely weak narrow diffraction peak at 2θ=10°, and the intensity of the low angle diffraction peak generally represents the length of the polymer side chain; There is a diffuse diffraction peak nearby 2θ=20°, with a interlayer spacing of approximately 0.35-0.39nm, which is a characteristic diffraction peak of long-chain olefins; No other diffraction peaks were found. The XRD spectrum of this product shows a diffuse scattering curve with low crystallinity, indicating that there are no rigid groups involved in the reaction process. It belongs to a flexible polymer and can well meet the requirements of low crystallinity for drag reducing agents. This low crystallinity polymer is easily soluble in oil, thereby improving drag reduction efficiency and duration. The XRD characterization results show that the synthesized product is a flexible α- Olefin polymer with low crystallinity.
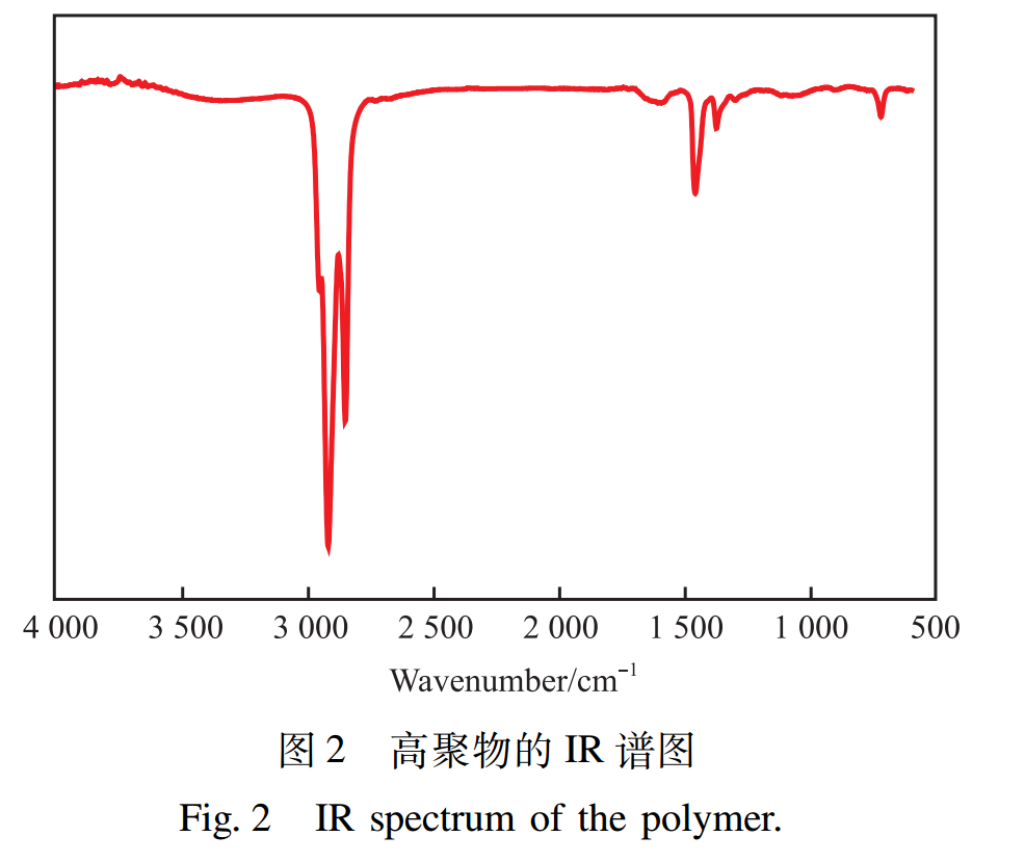
2.1.2 IR Characterization Results
The IR spectrum of the polymer is shown in Figure 2. As shown in Figure 2, a strong absorption peak appears at 2920.5,2852.4cm-1, which is attributed to the stretching and bending vibrations of the saturated C-H bond, respectively; The absorption peak at 1461.4cm-1 belongs to the stretching vibration of the C-C bond; The absorption peak at 1376.8cm-1 belongs to the bending vibration of the C-H bond in -CH3; The absorption peak at 722.4cm-1 belongs to the bending vibration of the C-H bond in - (CH2) n(n≤4). The IR characterization results show that the above absorption peaks are consistent with the characteristic absorption peaks of the main functional groups in the target product(α-hexylene /α-dodecene copolymer).
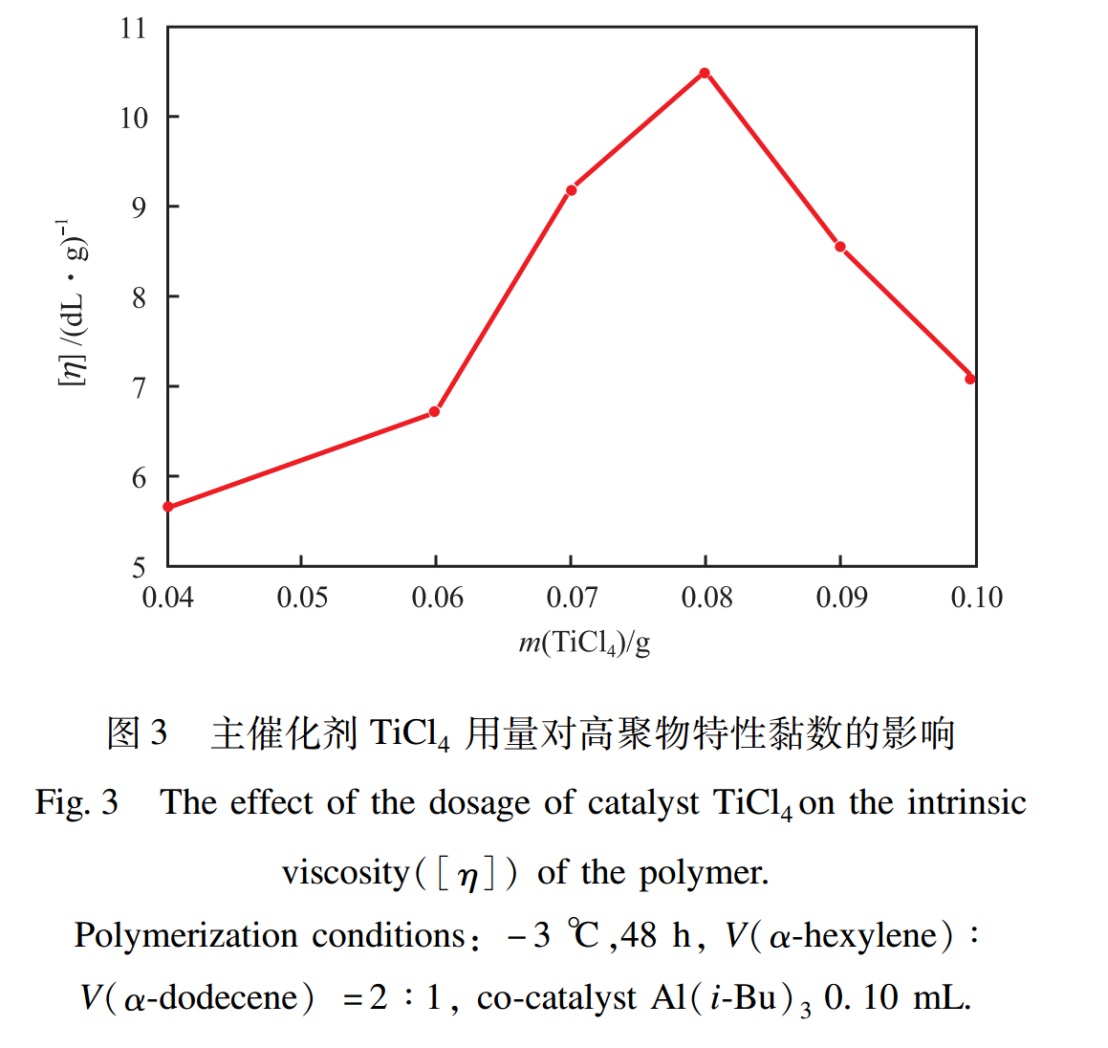
2.2 Effect of the Dosage of Main Catalyst TiCl4
The effect of the dosage of main catalyst TiCl4 on the intrinsic viscosity of polymers is shown in Figure 3. From Figure 3, it can be seen that the intrinsic viscosity of the polymer first increases and then decreases with the increase of TiCl4 dosage. When the TiCl4 dosage is 0.08g, the intrinsic viscosity reaches its maximum value (10.50dL/g). This is because when the amount of TiCl4 is low, Al (i-Bu)3 is excessive, in addition to forming active centers with TiCl4, the remaining Al (i-Bu) 3 can reduce TiCl4 to inactive TiCl3; When the amount of TiCl4 is too high, the excessive amount of TiCl4 increases the viscosity of the system, affects the diffusion of monomers, and hinders the mass transfer of the system, resulting in a significant decrease in the intrinsic viscosity. Therefore, it is more appropriate to control the dosage of TiCl4 at 0.08g.
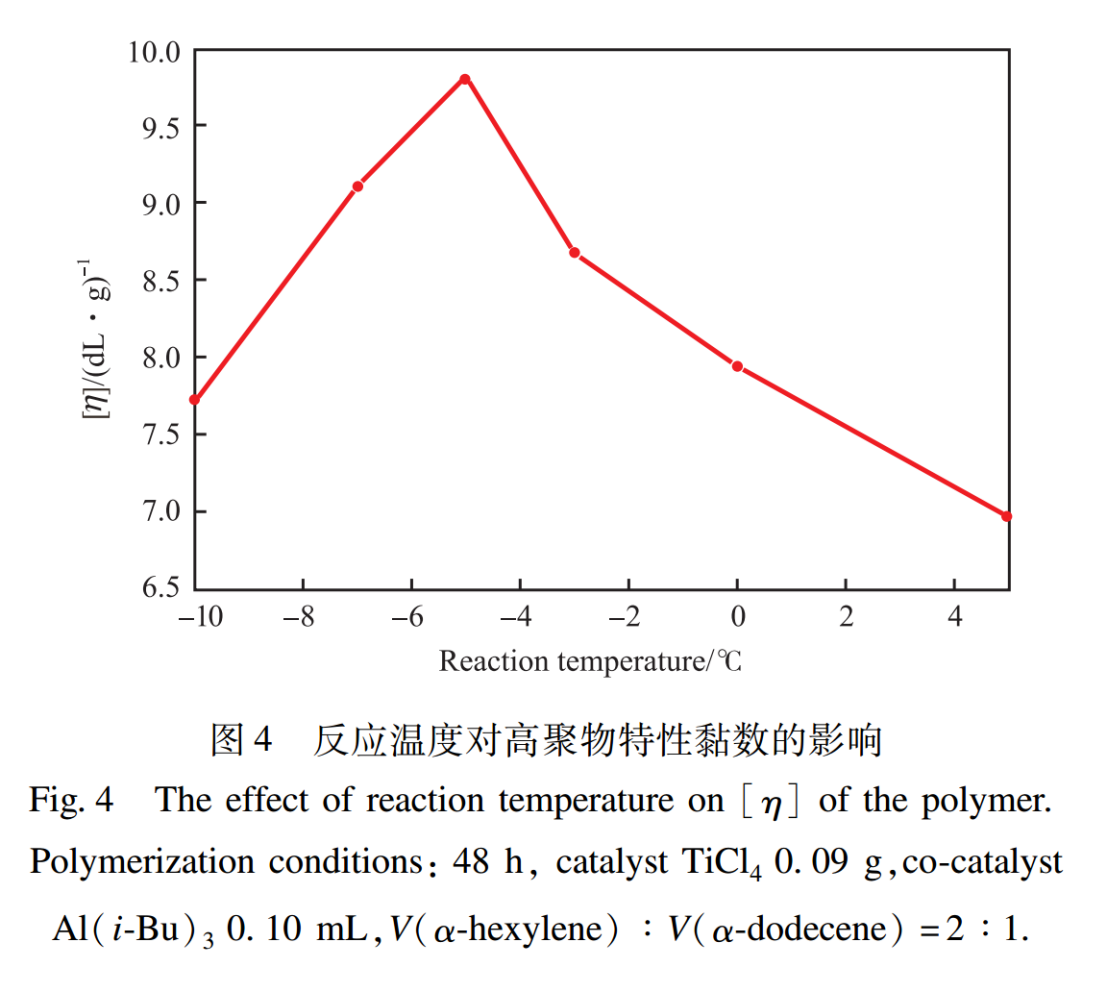
2.3 Effect of Reaction Temperature
The heterogeneous olefin coordination polymerization initiated by Ziegler-Natta catalyst is highly sensitive to reaction temperature, and the effect of reaction temperature on the intrinsic viscosity of the polymer is shown in Figure 4. From Figure 4, it can be seen that the intrinsic viscosity of polymers first increases and then decreases with the increase of reaction temperature. When the reaction temperature is -5℃, the intrinsic viscosity reaches its maximum value (9.63dL/g). This is because when the reaction temperature is too high, the reaction rate is too fast, which increases the viscosity of the system and easily leads to gel phenomenon; When the reaction temperature is too low, the reaction rate is too slow, and the monomer cannot fully polymerize within the reaction time. Therefore, it is more appropriate to choose a reaction temperature of -5℃.