Research and application status of temperature resistance polyacrylamide drag reducers(Part 1)
Abstract
Slick water fracturing is one of the main technical means of economic development for shale gas reservoirs,and polyacrylamide(PAM) drag reducer is the primary additive of slick water fracturing fluid. China has an abundance of deep shale gas resources,and the reservoirs are generally high temperature. PAM is susceptible to thermal degradation which leads to performance degradation. The development history of polymer drag reducers and the mechanism of temperature influence on the molecular stability of PAM drag reducers are reviewed. Then,three molecular design methods of temperature resistance PAM drag reducers were reviewed. Finally,the synthesis method of temperature resistance PAM drag reducer and its application in the fracturing deep shale gas wells are described. This paper aims to provide theoretical basis for the research of high temperature resistant fracturing fluids suitable for the development of deep oil and gas reservoirs.
China has abundant shale gas resources, with deep shale gas (buried at depths of 3500 to 4500 meters) accounting for over 65% of the total shale gas resources. It will undoubtedly become the main force in China's oil and gas exploration and development to increase reserves and production. Slick water fracturing is the main technical means for efficient development of shale reservoirs, and polymer drag reducing agents are the core additives of slick water fracturing fluids. Deep shale gas has the characteristics of large burial depth and high temperature (up to 155℃). Smooth hydraulic fracturing fluid generates a large amount of turbulence during the injection process, losing a large amount of kinetic energy that should have reached the reservoir, causing the fracturing fluid to fail to achieve its expected goals. The addition of polymer drag reducing agents can suppress the generation of turbulence and significantly reduce the friction generated by long wellbore during hydraulic fracturing.
Polymer drag reducing agents mainly consist of polyacrylamide (PAM), which has good thermal stability under anaerobic conditions and is less prone to breakage of molecular main chains at deep shale gas reservoir temperatures. However, when PAM is applied, it is mostly in an aqueous solution state, and the entry of dissolved oxygen in the air is inevitable during solution preparation, leading to the oxidation and degradation of PAM. High temperature can exacerbate this process, causing damage and fracture of PAM molecular chain structure, and significantly reducing the application performance of PAM. Therefore, the development and application prospects of temperature resistant PAM drag reducing agents are broad, and researchers mainly enhance the thermal stability of PAM by enhancing the rigidity of molecular chains.
This article reviews the research progress of polymer drag reducing agents, discusses the influence of temperature on the drag reducing effect of PAM, summarizes the research and application progress of temperature resistant PAM drag reducing agents, and introduces the technical status of PAM drag reducing agents suitable for high temperature reservoir slick water fracturing, in order to provide reference for the research and selection of fracturing fluids.
1. Overview of PAM Drag Reducing Agents
1.1 Development History of Polymer Drag Reducing Agents
Polymer drag reducing agents can be divided into natural plant gum drag reducing agents and synthetic polymer drag reducing agents based on their sources. Natural plant gum drag reducing agents mainly include guanidine gum, xanthan gum, coumarin gum, etc. In the 1860s to 1980s, guanidine gum and its derivatives (hydroxypropylation, carboxymethylation, cation, etc.) were mostly used in fracturing fluids. However, the drag reduction rate of natural plant gum is generally lower than 65%, which is not an ideal drag reducing agent.
In order to solve the problem of poor drag reduction effect of natural plant gum, researchers have conducted a large amount of research on synthetic polymer drag reducing agents. Synthetic polymer drag reducing agents mainly include polyethylene oxide, polyisobutylene, and PAM, among which PAM has the best drag reducing effect, with a drag reduction rate of over 70%. With the continuous deepening of research, low-cost and good drag reduction PAM has become the main research object.
1.2 Effect of Temperature on PAM Drag Reducing Agents
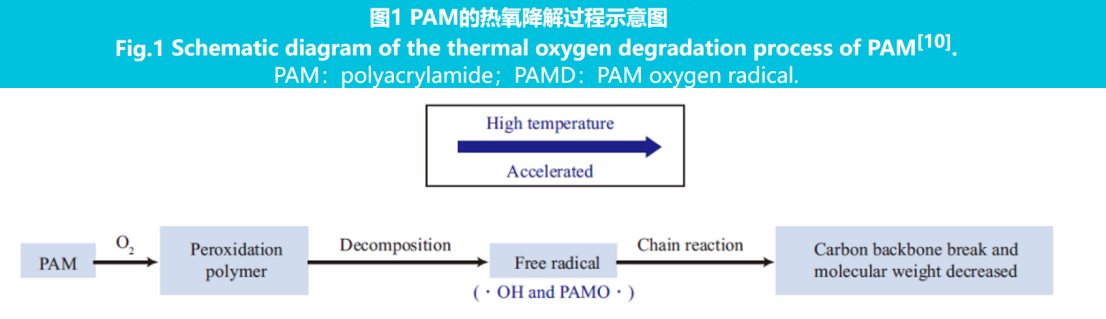
The high temperature of deep shale gas reservoirs puts forward higher temperature resistance requirements for fracturing fluids. The increase in reservoir temperature causes a synchronous increase in temperature in the wellbore, and the temperature resistance of drag reducing agents has become an important performance indicator of fracturing fluids. Thermogravimetric analysis found that PAM molecules began to degrade under anaerobic conditions at 326℃, indicating that pure PAM had good thermal stability. The thermal oxidative degradation process of PAM is shown in Figure 1.
As shown in Figure 1, PAM undergoes oxidative degradation under aerobic conditions, resulting in the breakage of the main chain of molecular carbon and a decrease in molecular weight. High temperature can accelerate the oxidative degradation of PAM. Zhu Linyong et al. found that PAM in aqueous solutions under a 90℃ air atmosphere undergoes significant degradation after 20 hours, resulting in a viscosity loss of over 80% of the PAM solution. Therefore, when using slick water for fracturing in high-temperature reservoirs, PAM drag reducing agents need to have good temperature resistance to maintain good drag reducing effects.
2. Research on Temperature Resistant PAM Drag Reducing Agent
The drag reduction performance of polymers in aqueous solutions is mainly related to their molecular weight, flexibility, chain structure, and solubility. The higher the molecular weight of the polymer, the stronger its molecular flexibility, the smaller the proportion of side groups, the stronger its water solubility, and the higher its degree of extension in water, the better its drag reduction effect.
With the increasing development scale of deep shale gas and other deep oil and gas reservoirs, drag reducing agents need to maintain good performance under high temperature conditions.
The methods to improve the molecular thermal stability of PAM drag reducing agents include:
1) . Synthesis of ultra-high molecular weight PAM;
2) . Copolymerization with temperature resistant monomers to improve molecular chain rigidity;
3) . Hydrophobic modification, utilizing intermolecular and intramolecular hydrophobic associations to enhance thermal stability;
4) . Nano material composite modification enhances molecular structural strength.
With the increase of molecular weight, the temperature resistance of PAM has been improved to a certain extent. However, the industrial production of ultra-high molecular weight PAM is difficult, and the molecular weight cannot be infinitely increased. The water solubility and shear resistance of PAM will also deteriorate with the increase of molecular weight, and the cost of raw materials will increase. Therefore, the synthesis of ultra-high molecular weight PAM is not an ideal method to improve the temperature resistance of drag reducing agents.
The molecular design of temperature resistant PAM drag reducing agents includes temperature resistant monomer copolymerization, hydrophobic monomer copolymerization, and inorganic nano/PAM composites.
2.1 Temperature Resistant Monomer Copolymerization
By copolymerizing acrylamide (AM) with temperature resistant monomers, large or rigid side groups can be introduced into the PAM molecular chain, increasing the motion resistance of the PAM molecular segment, weakening the thermal motion such as rotation and vibration inside the PAM molecule, increasing the rigidity of the molecular chain, and improving the thermal stability of PAM. The commonly used temperature resistant comonomers are monomers containing sulfonic acid groups and monomers containing cyclic structures.
2.1.1 Monomers containing sulfonic acid groups
- SO3- is a strongly polar group with good thermal stability and is not sensitive to high temperatures, which can improve the rigidity and water solubility of PAM molecular chains. The commonly used sulfonic acid containing monomers are 2-acrylamide-2-methylpropane sulfonic acid (AMPS), sodium styrene sulfonate (SSS), sodium vinyl sulfonate, sodium propylene sulfonate, etc. Currently, most research reports are about AMPS.
The AM/AMPS copolymer molecular chain contains large side groups, which increases the steric hindrance and further enhances the rigidity of the PAM molecular chain, making the AM/AMPS copolymer have good temperature resistance. However, the improvement of temperature resistance of AMPS on PAM is limited, as the amide group on AMPS undergoes hydrolysis with increasing temperature, leading to the detachment of sulfonic acid groups and reducing the stability of AM/AMPS copolymer molecular chains. Some researchers have proposed using AMPS to enhance the temperature resistance of PAM at temperatures below 93℃.
Compared with AM/AMPS copolymers, the side groups of AM/SSS copolymers contain benzene rings, while the stability of the benzene sulfonic acid side group is better but the water solubility is poor. Borai et al. prepared AM/SSS copolymers through radiation induced template polymerization, proposing that hydrogen bonds can be formed between sulfonic groups and carbonyl and amine groups in AM, thereby forming association structures within and between PAM molecules, enhancing the intermolecular forces of the polymer and enhancing the stability of PAM.
2.1.2 Monomers with circular structure
The commonly used monomers with cyclic structures are N-vinylpyrrolidone (NVP), chitosan, and vinyl β- Cyclodextrin and acryloyl morpholine (ACMO), etc. The introduction of a circular structure into the side groups of PAM molecules increases the steric hindrance of molecular chain segment movement, reduces the number of conformations generated by the internal rotation of C-C single bonds, and locally becomes rigid, improving the thermal stability of PAM molecular chains. The most commonly used monomer with circular structure is NVP. Xu et al. used thermogravimetric and infrared spectroscopy techniques to study the thermal stability of AM/NVP copolymers. When the temperature is below 300 ℃, NVP can effectively inhibit the hydrolysis of amide groups, and hydrogen bonds are formed between the carbonyl and amide groups in NVP, enhancing the stability of PAM molecular chains. Moradi Araghi et al. compared the thermal stability of 2-AM-2-methylpropylsulfonic acid sodium (AM/NaAMPS) copolymer and AM/NVP copolymer at 121 ℃ and found that the AM/NaAMPS copolymer fully hydrolyzed after 30 days of aging, while the hydrolysis degree of the AM/NVP copolymer was about 80% after 100 days of aging. The experimental results indicate that the thermal stability of AM/NVP copolymer is better.
The copolymerization of AM with temperature resistant monomers increases the resistance of molecular chain segment motion, weakens the thermal motion within and between molecules, improves the rigidity of molecular chains, and enhances the temperature resistance of PAM. When designing the molecular structure of temperature resistant monomers, attention should be paid to:
1) . The first choice for industrial production and application is water-soluble and temperature resistant monomers;
2) . The drag reduction effect of PAM is positively correlated with the flexibility of the molecular chain. While improving the rigidity of the molecular chain, consideration should be also given to the flexibility of the molecular chain;
3) . A single temperature resistant monomer may not be able to meet the needs of temperature resistance and drag reduction at the same time, and multiple temperature resistant monomers can be considered for multicomponent copolymerization.
We (Dico Energy) has a series of pipeline drag reducing agent products, and the detailed information of each product can be found on our company's website www.dicoenergy.com in Drag Reducer.
