Research and Application Progress on Nanofluid Enhanced Oil Recovery (Part 1)
Abstract
In response to the problems of low viscosity retention and high adsorption loss in traditional solutions such as polymers and surfactants in improving crude oil recovery, this article introduces the research progress of nanofluids in improving crude oil recovery. This article summarizes the synthesis methods of nanomaterials and evaluation methods for the stability of nanofluids currently used in the field of improving crude oil recovery; Six main mechanisms of nanofluids for enhancing oil recovery are summarized, including reducing interfacial tension, changing wettability, reducing oil viscosity, improving foam stability, structural separation pressure, and reducing pressure and increasing injection; This article investigates the current progress in the field application of nanofluids to improve oil recovery in oil fields, and proposes the bottleneck problem of limiting the large-scale application of nanofluids in oil fields. Firstly, there is a lack of efficient nano oil displacement systems for developing unconventional reservoirs; The second is that the theoretical and technical research on the development of two-dimensional sheet-like nanofluids, the mechanism of improving oil recovery, and the integration of field pilot experiments is not yet systematic, and requires deeper exploration and research. This article points out the direction for the practical promotion and application of nanofluids.
Since the 21st century, nanotechnology has been widely applied in various industries, such as medicine, food, electronics, oil and gas, etc. Nanotechnology refers to the application of solid materials with at least one dimensional size in the range of 1-100 nm in engineering research. Richard Feynman first introduced the idea of nanotechnology at the American Physical Society held at the California Institute of Technology on December 29, 1959, titled "There is enough space at the bottom.". In recent decades, nanotechnology has developed rapidly, and research in the oil and gas industry has also increased. Nano oil displacement technology is one of the very important research directions. There are many nanomaterials used to improve crude oil recovery, which can be divided into spherical nanoparticles (SiO2, TiO2, Al2O3, CuO, ZnO, etc.) and two-dimensional sheet-like nanoparticles (graphene oxide, silicon-based nanosheets, molybdenum disulfide, etc.) according to their shapes; According to surface properties, they can be divided into single wettable nanoparticles and amphiphilic (Janus) nanoparticles. By dispersing different nanoparticles in different dispersion media, different nanofluids can be constructed, and different nanofluids will produce different oil increasing mechanisms and oil displacement effects in porous media of oil reservoirs. Based on a large amount of domestic and foreign literature research, this article summarizes the synthesis methods of relevant nanomaterials applied in the field of improving crude oil recovery, introduces the commonly used evaluation methods of nanofluid stability, elaborates on the six main mechanisms of nanofluid improving crude oil recovery, investigates the current examples and effects of nanofluid application in oil fields, and finally, in view of the current research progress and application status of nanofluid improving crude oil recovery, combined with years of research results, shares the understanding of the future development potential of nanofluid improving crude oil recovery.
1. Synthesis of Nanoparticles
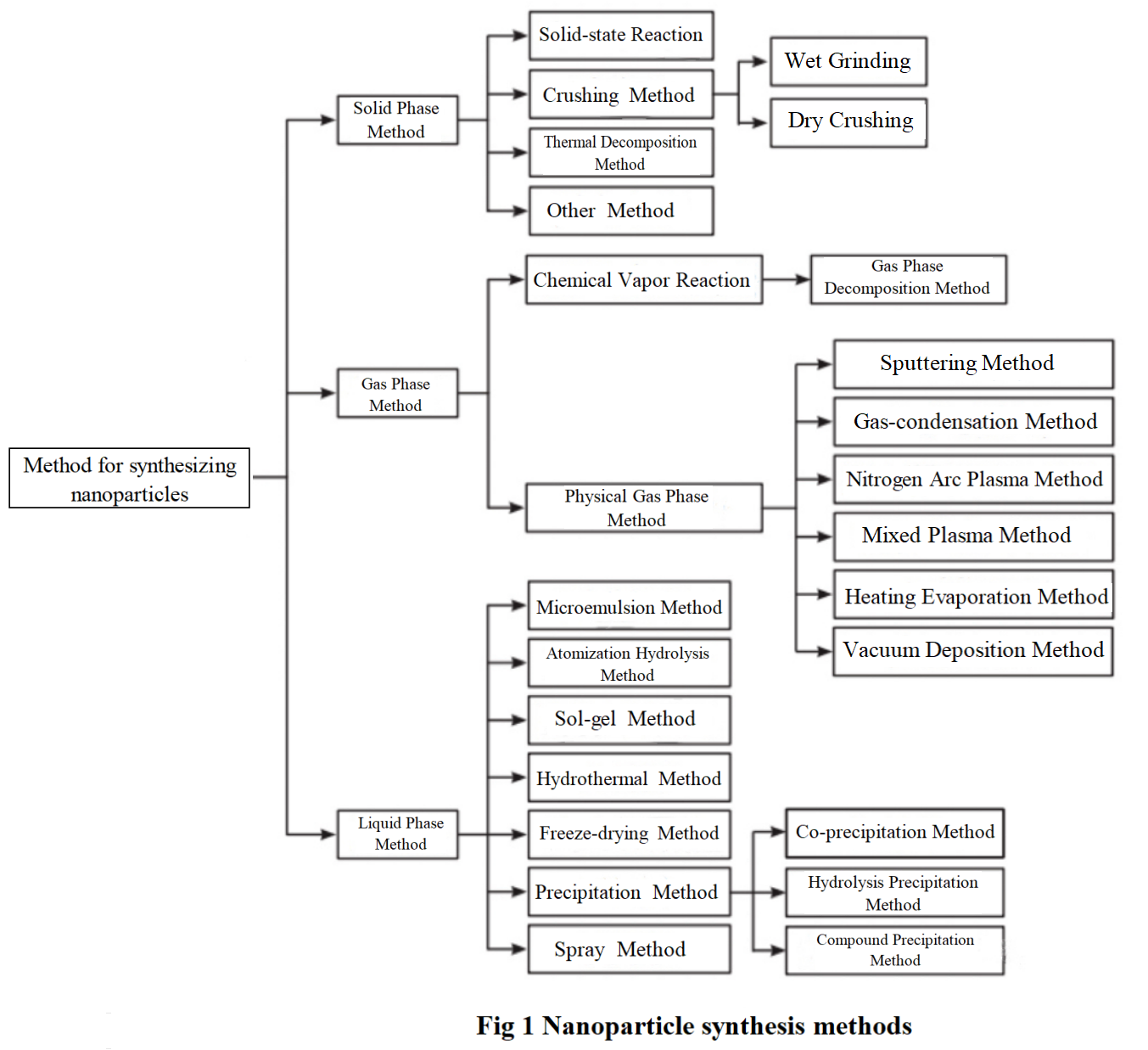
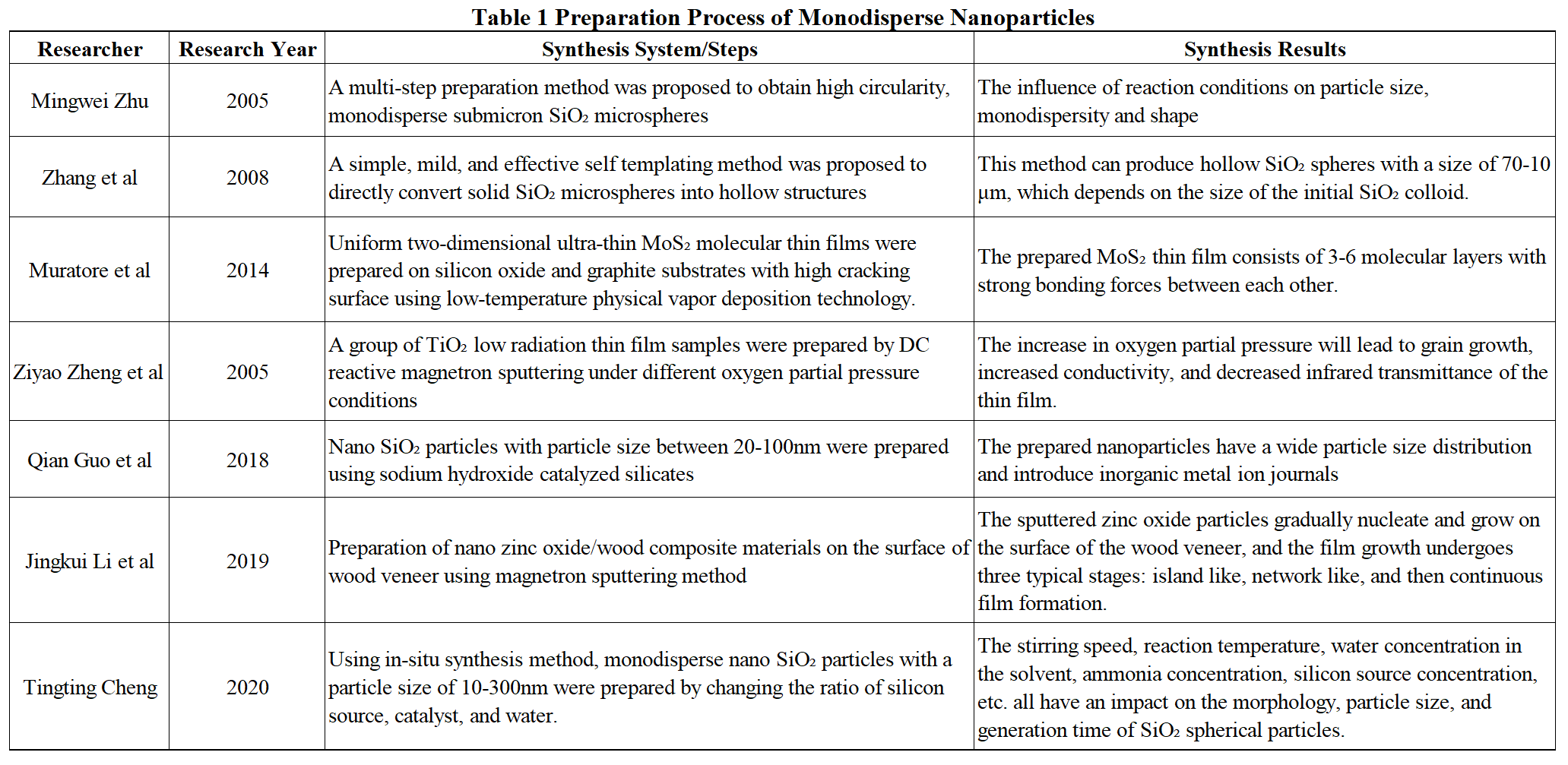
One of the biggest challenges in the development of nanomaterials is the preparation of materials. There are various methods for preparing nanomaterials, which can be divided into solid-phase method, liquid-phase method, and gas-phase method according to the material state; According to the preparation methods, it can be divided into physical methods, chemical methods, and biological methods. The synthesis methods of nanomaterials are summarized in Figure 1. Table 1 summarizes the synthesis process of monodisperse nanoparticles by different scholars.
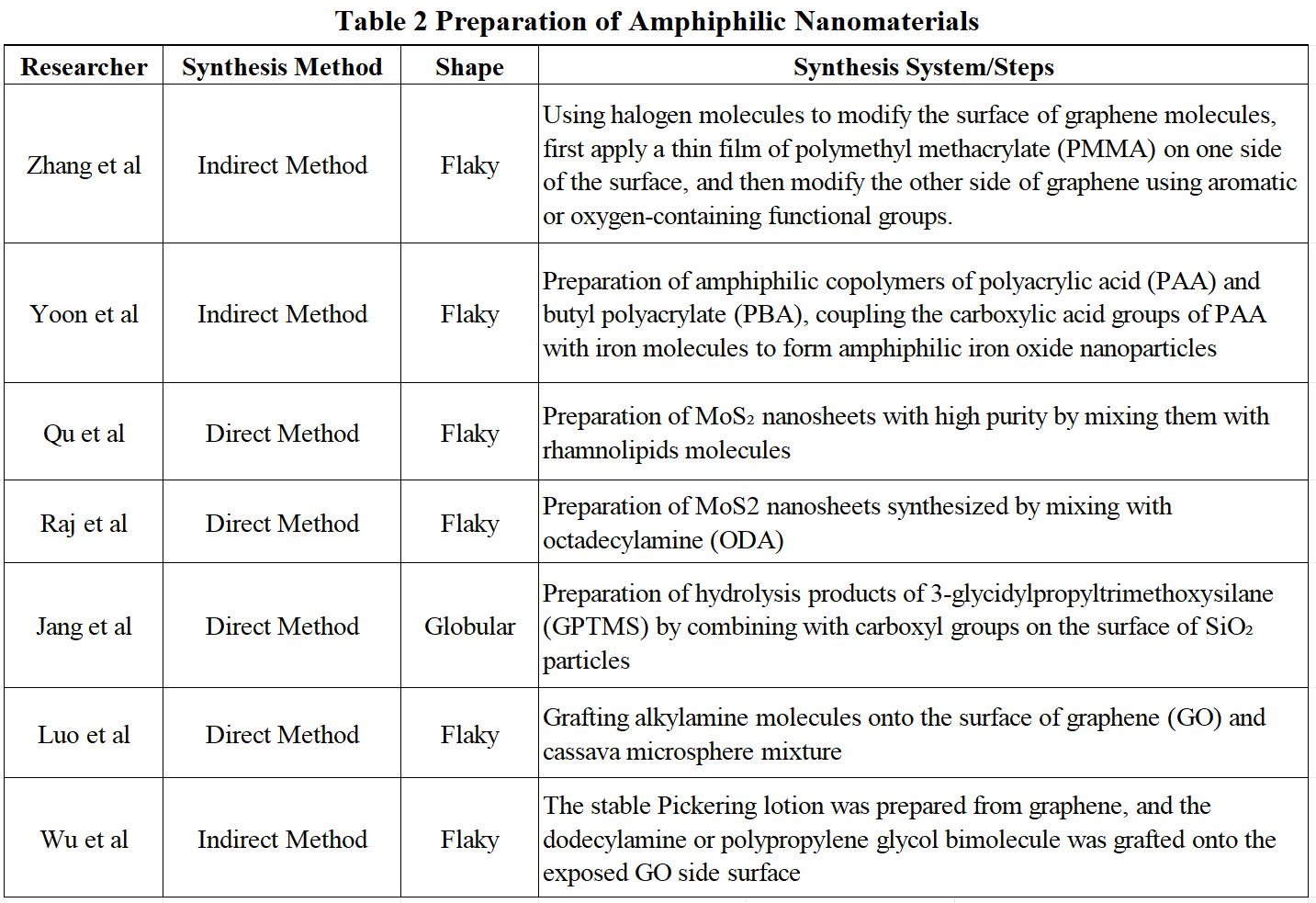
In recent years, scholars have found that amphiphilic nanomaterials have better properties than monophilic nanomaterials, and are receiving increasing attention in fields such as environmental protection, food safety, and energy. Amphiphilic nanomaterials can also be divided into spherical, sheet and rod shapes. Studies have found that the interfacial activity of amphiphilic nanomaterials is stronger than that of spherical and rod nanomaterials. Once adsorbed to the interface, more energy is required to desorb them from the interface, so they can be used to improve the stability of emulsions and foam, thus improving crude oil recovery. According to the research summary and analysis of relevant literature at home and abroad in recent years, the synthesis methods of amphiphilic nanomaterials mainly include direct and indirect methods, as shown in Table 2.
2. Stability of Nanofluids
Nanofluid refers to the dispersion of nanomaterials in specific dispersants to form a special suspension. To ensure that nanofluids can effectively enhance oil recovery in geological environments, it is necessary to explore and study their stability. At present, there are many methods and means to study the stability of nanofluids, with the most commonly used being the Zeta potential method, pH control method, and precipitation method.
2.1 Zeta Potential Method
Due to the same charge on the surface of nanoparticles, there must be electrostatic repulsion between adjacent nanoparticles. The Zeta potential is composed of van der Waals forces and electrostatic repulsion, and its value can accurately reflect the stability of nanofluids. Cacua et al. found that the higher the absolute value of Zeta potential, the greater the repulsion between particles, and the better the stability of the nanofluid. Scholars have found that when the absolute value of Zeta potential is greater than 30 mV, the stability of its suspension is better. Kim et al. prepared a nanofluid containing Au, and measured that the Zeta potential value of the nanofluid was negative, and the absolute value of the Zeta potential was large, indicating strong repulsion between nanoparticles. The Au nanofluid has good stability. Souza et al. found that in order to increase the stability of magnetic graphene nanofluids, the Zeta potential of the nanofluid needs to be maintained at around 41.3 mV. In addition, inorganic salt ions with different valence states at the same concentration can also have an impact on the Zeta potential, with the order of influence being trivalent ions>divalent ions>monovalent ions.
2.2 pH Control Method
The pH control method is to regulate the pH value of the nanofluid to form a thick diffusion double electron layer on the surface of the nanoparticle, thereby improving the stability of the nanofluid. Zhang et al. found that when the pH value of 0.25 vol% TiO2 nanofluid was 12, the system was stable for 30 days without precipitation. Katiyar et al. investigated the dispersion stability of Al2O3, Bi2O3, and Al nanoparticles in a mixture of deionized water, deionized water, and ethanol. The results showed that temperature, dispersion type, and nanoparticle concentration all had an impact on the pH value of the system. At a certain temperature, as the concentration of nanoparticles decreases, the pH value of the nanofluid tends to be acidic, while conversely, it will remain consistent with the pH value of the dispersion. Wang Chao studied the effect of pH value on the stability of zinc oxide nanofluids and found that when pH value is 8, the upper clear liquid height of zinc oxide nanofluids is 1 mm, and the dispersion stability is the best.
2.3 Precipitation Method
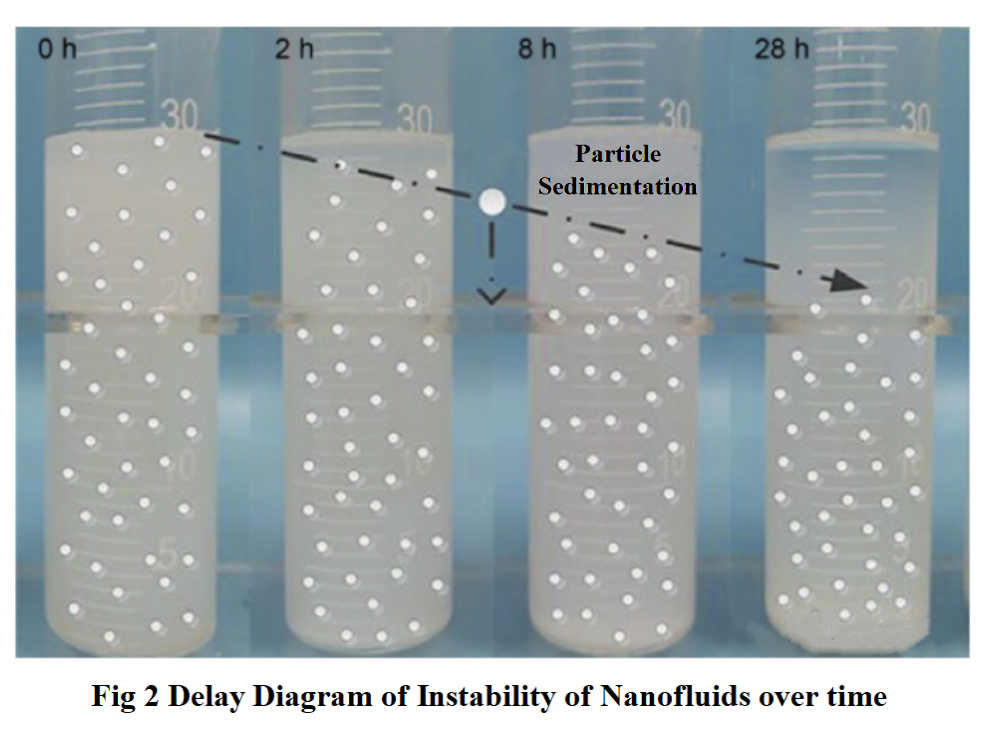
The precipitation method is a direct method for evaluating the stability of nanofluids. This method observes whether there is a layering phenomenon between the nanoparticles and the dispersion, and records dynamic photos during the layering process as a judgment indicator for the stability of nanofluids, as shown in Figure 2. However, the precipitation method cannot determine the concentration of local nanoparticles in the nanofluid, and can only evaluate the stability of the nanofluid from a macroscopic perspective. Sun et al. evaluated the stability of Cu and Al2O3 nanofluids using precipitation method. The experiment found that the stability of nanofluids is related to the particle size of nanoparticles. As the particle size increases, the stability of nanofluids gradually decreases and the settling speed also gradually increases.