Research Progress of Hyperbranched Polyethylene New Materials(Part 1)
Abstract
Due to its different topological structure from traditional polyethylene, hyperbranched polyethylene exhibits many unique physical properties. The synthesis and application research progress of hyperbranched polyethylene new materials were reviewed from three aspects: polyolefin elastomers, synthetic lubricants, and nanomaterial dispersants. The advantages and existing problems of developing hyperbranched polyethylene new materials were analyzed.
Polyolefin materials play an irreplaceable role in improving people's production and living standards, and promoting the development of new technologies. Polyethylene material is the largest variety of polyolefin materials, widely used due to its excellent mechanical properties, chemical stability, superior processing performance, reusability, and low cost. After more than 20 years of development, α-Diimine nickel palladium catalyzed ethylene chain walking polymerization has been able to achieve controllable preparation of hyperbranched polyethylene (HBPE). The different topological structures make this type of polyethylene material have many unique physical properties, which is expected to increase the added value of polyethylene materials. Although the relevant chain walking polymerization mechanism and catalyst design theory have been extensively and deeply studied, the application and industrialization of HBPE new materials have encountered bottlenecks.
This article reviews the current research status of the application of HBPE new materials, mainly including their applications in polyolefin elastomers, synthetic lubricants, and nanomaterial dispersants. On this basis, the advantages and existing problems of developing HBPE new materials were analyzed, attempting to promote the development of HBPE new material applications.
1. HBPE Elastomer
Conventional polyolefin elastomers typically require at least two monomers to be copolymerized, one to obtain a thermoplastic hard segment and the other to form a rubber like soft segment. Such as ethylene propylene rubber, ethylene/ α-Random copolymer of olefins (especially ethylene/1-octene copolymer), ethylene/ α-Olefin multi block copolymers, especially ethylene/1-octene multi block copolymers, have excellent mechanical and processing properties and have been widely used in daily life and industrial fields. Since Professor Brookhart used α-diimide nickel palladium as a catalyst for ethylene polymerization to obtain HBPE in 1995, people have been paying attention to the performance of this new type of polyethylene, as this technology can directly prepare products with the structure of traditional ethylene/α-Olefin copolymer through chain walking homopolymerization using a single ethylene as raw material. However, due to the poor mechanical strength of the early obtained HBPE material, research on its use as an elastomer was interrupted.
The early domestic research on the mechanical properties of HBPE was conducted by Zhang Danfeng's research group, who utilized the classic α-Diimine nickel catalyst A (see Figure 1) synthesized HBPE under high pressure, with the melting point and glass transition temperature of the product ranging from 61 to 101℃ and -62 to -49℃, respectively. The tensile strength of the obtained HBPE material reaches 27.9 MPa, and the elongation at break is as high as 774.6%. There is no obvious yielding phenomenon during the stretching process.
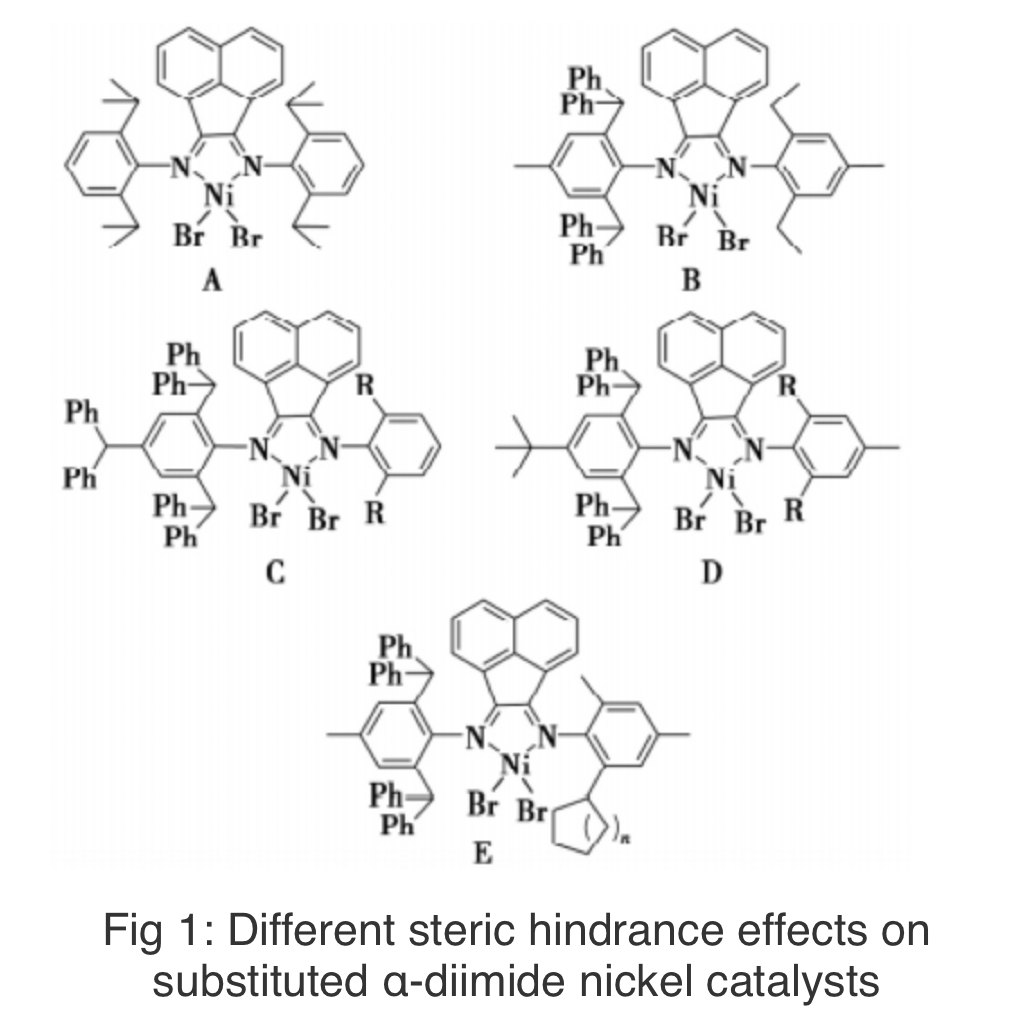
Afterwards, Sun Wenhua's research group conducted research on the preparation and physical properties of HBPE elastomers. They designed and synthesized a B-type α-diimide nickel catalyst (see Figure 1), synthesized a series of randomly hyperbranched linear polyethylene at different temperatures, and studied its thermal and mechanical properties. The results showed that as the polymerization temperature increased, the crystallinity of the product significantly decreased. The tensile strength of the product HBPE obtained at 40℃ could reach 4.8 MPa, while the mechanical properties of the product HBPE obtained at 80℃ were very poor. The product HBPE obtained at 40℃ exhibits an elastic recovery rate of up to 80% at a strain of 250%. They pointed out that although HB-PE has a high degree of branching, a large number of linear segments are randomly distributed on the main chain and can serve as physical cross-linking points for hard segments. High branched segments spaced between linear segments can serve as soft segments, thus forming the required soft hard substitution structure for elastomers.
Recently, Sun Wenhua's research group has synthesized three new asymmetric α-diimide nickel catalysts C~E (see Figure 1), and studied the performance of the resulting polyethylene elastomers. The C-type catalyst has excellent thermal stability, and its activity can still reach 2.97×106g/mol·h at 90℃. With the increase of polymerization temperature (20℃ to 40℃), the tensile strength decreases from 13.52MPa to 6.25MPa, the elongation at break increases from 218.3% to 518.9%, and the maximum elastic recovery rate can reach 84%. Using D-type catalysts, ultra-high molecular weight branched polyethylene with a heavy average molecular weight of up to 3.08 million can be obtained. With an elongation at break of up to 843.9%, the elastic recovery rate can still reach 69%, which has good application prospects in thermoplastic elastomers. They studied the effects of ring size and reaction temperature on ethylene polymerization of E-type catalysts with different aliphatic ring unilateral substitutions, and conducted performance tests on polyethylene obtained from catalysts substituted with cyclooctane. The results showed that the comprehensive mechanical properties of the product HBPE obtained at 40℃ were the best when using sesquiethyl aluminum chloride as a co catalyst (tensile strength of 4.8 MPa, elongation at break of 590%); When modified methylaluminoxane is used as a co catalyst, the comprehensive mechanical properties of the product HBPE obtained at 30℃ are the best (tensile strength is 5.7MPa, elongation at break is 481%).
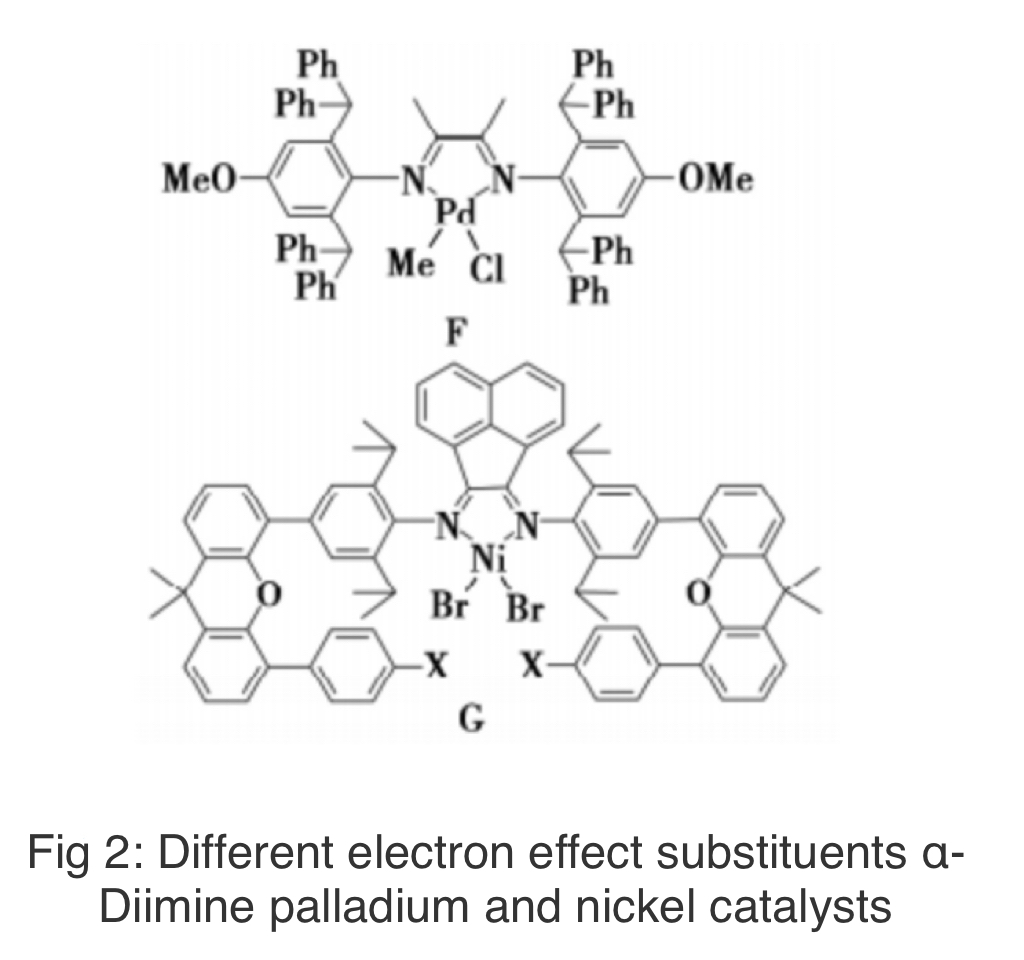
In addition, the Chen Changle research group also conducted a preliminary study on the mechanical properties of HBPE obtained from the α-diimide nickel palladium catalyst (Figure 2). Firstly, a α-diamine palladium catalyst F with electron donating effect was synthesized. The tensile strength of HBPE obtained at 101.3kPa and 911.7kPa was around 15MPa, and there was no obvious yielding phenomenon. However, the elongation at break of polyethylene obtained at atmospheric pressure was much higher than that of high-pressure products. Then they synthesized a series of α-diimide nickel catalysts G with different electronic effects (see Figure 2), and the tensile strength and elongation at break of the obtained polyethylene elastomer can be adjusted in the range of 0.2~26MPa and 300%~1400%, respectively, with good elastic recovery rate.
The reason why HBPE has the properties of an elastomer may be due to its high molecular weight and high content of long branched chains. Recently, Pellecchia's research team used the classic A-type α-diimide nickel catalyst to achieve the regulation of polyethylene microstructure through different alkyl aluminum activations, and also obtained polyethylene elastomers. Pellecchia believes that compared to ethylene propylene copolymers, HBPE can achieve the transition from plastic to elastic at a lower branching degree, which is similar to ethylene/long chain α-Olefin copolymers.
The above research results indicate that chain walking polymerization can catalyze ethylene homopolymerization to directly prepare polyolefin elastomers, thereby increasing the added value of polyethylene materials. In addition, the design of the α-diimide nickel palladium catalyst structure can also achieve the regulation of the elastic mechanical properties of HBPE. These research results have laid an experimental and theoretical foundation for the practical application of HBPE in polyolefin elastomers to a certain extent.
2. HBPE Lubricating Oil
Currently, polyolefin lubricants (PAO) are typically synthesized from high-grade α-olefins using Lewis acid, metallocene, ionic liquids, or chromium salts as catalysts for oligomerization, resulting in high-performance lubricants such as PAO-6, PAO-10, and PAO-20.
Poly α-olefin synthetic lubricants have excellent viscosity temperature properties (high viscosity index, VI ≥ 120) and low-temperature flowability (low pour point, PP ≤ -30℃), but the high raw material price (1-decene or other advanced α-olefins) directly drives up the price of polyolefin synthetic oils, limiting the promotion and application of PAO. In addition, at present, polyolefin lubricants mainly face problems such as complex process flow (three step catalytic reaction: ethylene oligomerization, α-olefin low polymerization, catalytic hydrogenation, and high-temperature steam reduction classification), low target product yield (low selectivity), high energy consumption, and high pollution, resulting in high production costs. If cheap raw materials such as propylene, butene, and even ethylene can be used to directly produce polyolefin lubricating oil through oligomerization through the development of new catalysts, it can not only greatly simplify the synthesis process, reduce production costs, but also reduce environmental pollution and increase the added value of polyethylene materials.
Professor Brookhart's research found that the α-diimide palladium catalyst has strong chain walking ability and is easy to form branched chains. Therefore, even if the molecular weight of the obtained HBPE is very high (105g/mol), the product is usually oily. Therefore, people began to study the direct synthesis of ethylene into polyolefin lubricating oil, but progress was very slow due to technical difficulties. Although α-Palladium diimide catalyzed ethylene chain walking polymerization can yield hyperbranched and oil-like polyethylene products, and the product HBPE has a high viscosity index, the viscosity of the product is too high, and the low-temperature flowability is poor (pour point is too high), making the early obtained HBPE can only be used as a viscosity index improver for lubricating oil, and cannot be directly used as a synthetic lubricating oil.
The Ye research group was the earliest to conduct research on the synthesis and application of HBPE lubricating oil. They used the classic α-diimide palladium catalyst H (see Figure 3) to synthesize a series of medium to low molecular weight (500-25kg/mol), oil-like HBPE under low pressure, and conducted a detailed analysis of its rheological properties, glass transition temperature, melting point, etc. The research results indicate that the obtained HBPE oil has a lower glass transition temperature (-69 ~ -80℃), an adjustable kinematic viscosity in the range of 1.9~460mm2/s at 100℃, and a viscosity index of 92~298, indicating that the product has good prospects for lubricating oil applications.
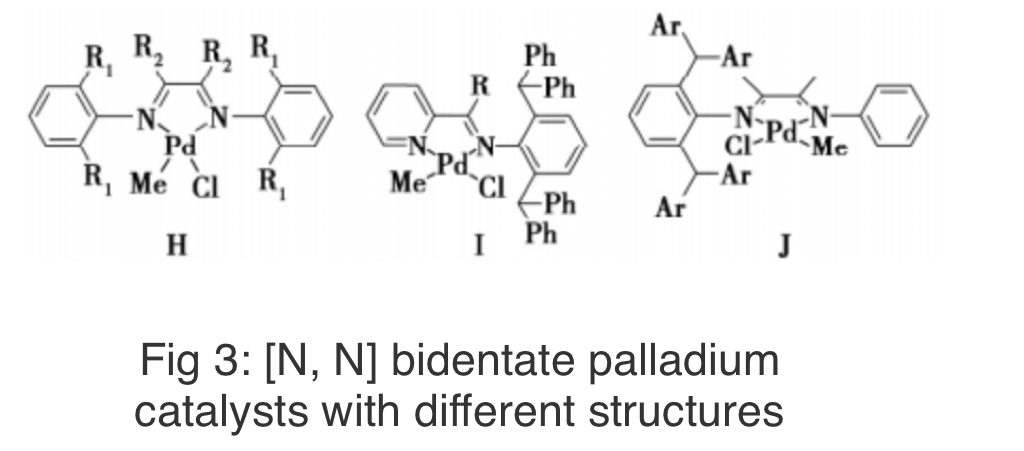
In our previous work, we designed and synthesized two ortho diphenylmethyl substituted pyridine imine palladium catalysts I, which were used to catalyze ethylene polymerization under low pressure to obtain ultra-low molecular weight (580-760g/mol), low viscosity, and oil-like HBPE (branching degree 110-210/1000C). The introduction of diphenylmethyl greatly improved the thermal stability of the catalyst, and the catalytic activity remained unchanged for 12 hours at 45℃.
In addition, Guo Lihua's research team has designed and synthesized various asymmetric α-diimide palladium catalysts J containing substituted diphenyl methyl groups, which can also efficiently catalyze ethylene oligomerization and achieve macro scale preparation of HBPE. Although oil-like HBPE has been successfully prepared, no tests have been conducted on its viscosity temperature performance and low-temperature flowability.
