Research Progress in Chemical Viscosity Reduction Technology for Medium to Deep Heavy Oil (Part 4)
Although oil soluble catalysts can enhance their activity through more thorough contact, there is still a problem of catalyst oil phase separation. Therefore, researchers have proposed an amphiphilic catalyst that combines the advantages of water solubility and oil solubility. Chen et al. designed and synthesized an amphiphilic metal chelate - iron arylsulfonate, which improved the dispersion of cations in oil and helped the catalyst maintain stability at the oil-water interface. The experimental results showed that the apparent viscosity of heavy oil decreased by 90.7% at 200℃. In on-site testing, not only did the viscosity decrease by 82.3%, but the stability also significantly improved. Subsequently, Chen et al. synthesized a novel Gemini catalyst with transition metals as active centers and Gemini surfactants as ligands. At 170℃, the viscosity decreased by more than 90% in both laboratory and field tests.
Another important type of catalyst is heterogeneous catalysts, including solid acids and natural zeolites. Strausz et al. used tetrafluoroboric acid (HF·BF3) as an active catalyst for hydrothermal cracking of heavy oil rich in asphalt, tar sand, and asphaltene. The results showed that through depolymerization and hydrogenation reactions, the conversion rate was as high as 56%, and the main products were volatile organic compounds and alkylbenzene. Although HF·BF3 has a significant effect in reducing viscosity and improving quality, its water solubility will bring about subsequent recovery issues. Therefore, researchers have begun to develop other solid catalysts such as heteropolyacids and modified zirconia. Wen Shoubin et al. used H4SiW12O40 for viscosity reduction of heavy oil in Shengli Oilfield. After 36 hours of reaction at 240℃, the viscosity decreased by more than 67%. And when H4SiW12O40 interacts with reservoir minerals, the viscosity further decreases to 73%. Chen et al. found that Keggin type nano K3PMo12O40 and H3PMo12O40 can significantly reduce the viscosity of ultra heavy oil due to their unique characteristics in acidity and redox reactions. Jing et al. used Ni2+and Sn2+to modify SO42-/ZrO2 and demonstrated that modified SO42-/ZrO2 can catalyze the hydrothermal cracking reaction of heavy oil in Shengli Oilfield, significantly reducing the content of resin, asphaltene, sulfur, and nitrogen. In addition, natural zeolite is an economically abundant mineral resource, and can also be used as a catalyst when appropriately activated with a certain acidity. Junaid et al. used zeolite and clinoptilolite as catalysts and concluded that natural zeolite can effectively remove impurities. Researchers also found that the porous structure of zeolite can adsorb excess components, and the acidity of zeolite can provide a large amount of hydrogen ions to stabilize intermediate cracking molecules, which is a completely different ion mechanism from the hydrogenation cracking mechanism.
In recent years, nanomaterials have attracted increasing attention. Due to the unique characteristics of nanoscale materials, such as high catalytic activity, high specific surface area, and effective transport within porous media, nanocatalysts are gradually being developed and applied in heavy oil hydrothermal cracking. Li Wei et al. prepared nano nickel micro lotion and applied it to the hydrothermal cracking of Liaohe super heavy oil. The research shows that nano Ni micro lotion can promote desulfurization, asphaltene conversion and reduce viscosity. Under the synergistic effect of quality improvement, emulsification, and dilution, the viscosity reduction rate of asphalt at 50℃ reaches 98.9%. In addition to a decrease in viscosity, the H/C ratio of the modified heavy oil significantly increases, while the resin, asphaltene, and sulfur content significantly decrease. Noorlaily et al. attempted to use microwave assisted co precipitation method to prepare NiO nanoparticles as catalysts for heavy oil hydrothermal cracking. The author synthesized NiO spherical nanoparticles with an average particle size of 65 nm and an average specific surface area of 158 m2/g using NiCl2·6H2O as the nickel source. The results of catalyst activity testing indicate that adding NiO nanoparticles in the hydrothermal cracking reaction can reduce the viscosity of heavy oil by 22%. Li et al. used carbon nanomaterials instead of transition metal catalysts to reduce the viscosity of heavy oil by over 96% in a short period of time at a low temperature of about 150℃, ensuring the viscosity reduction effect while also saving operating costs. Hashemi et al. reported the micro lotion containing three metal (W, Ni and Mo) colloidal nanoparticles as a catalyst, and proved that they can enhance the recovery of asphaltene. In order to elucidate the potential mechanism, Shokrlu et al. investigated the effects of metal type, size, and concentration of nanoparticles on catalytic activity, and believed that the above parameters are very important and should be combined and optimized to achieve better performance.
It is undeniable that hydrothermal catalytic cracking technology is a promising technology for improving heavy oil recovery. It transforms heavy oil reservoirs into underground refineries, while achieving crude oil upgrading and recovery. Transition metal salts, transition metal organic compounds, and other water-soluble catalysts are still the mainstream of catalysts, but their widespread application in heavy oil development is limited by issues such as catalyst recovery, environmental impact, and high cost. Super dispersed nano catalysts will become a future research focus due to their excellent properties such as heat resistance, recyclability, and multiple reactive active sites. However, further determination of the optimal reaction conditions and mechanism for nanoparticle catalysis is needed. Meanwhile, the synthesis methods of some nanoparticles are too complex for on-site applications, and further optimization of the synthesis process is needed.
3.4 Nanomaterial viscosity reduction
As mentioned earlier, nanoparticles have great potential for application in the hydrothermal catalytic cracking of heavy oil due to their high catalytic activity. In fact, nanoparticles have strong adsorption properties and strong affinity with asphaltene, which can accelerate reaction rates such as oxidation and gasification. Therefore, nanoparticles can decompose asphaltene under different conditions, which also means that the application of nanotechnology can effectively improve the efficiency of almost all thermal recovery technologies (such as ISC, CSS, SAGD, etc.).
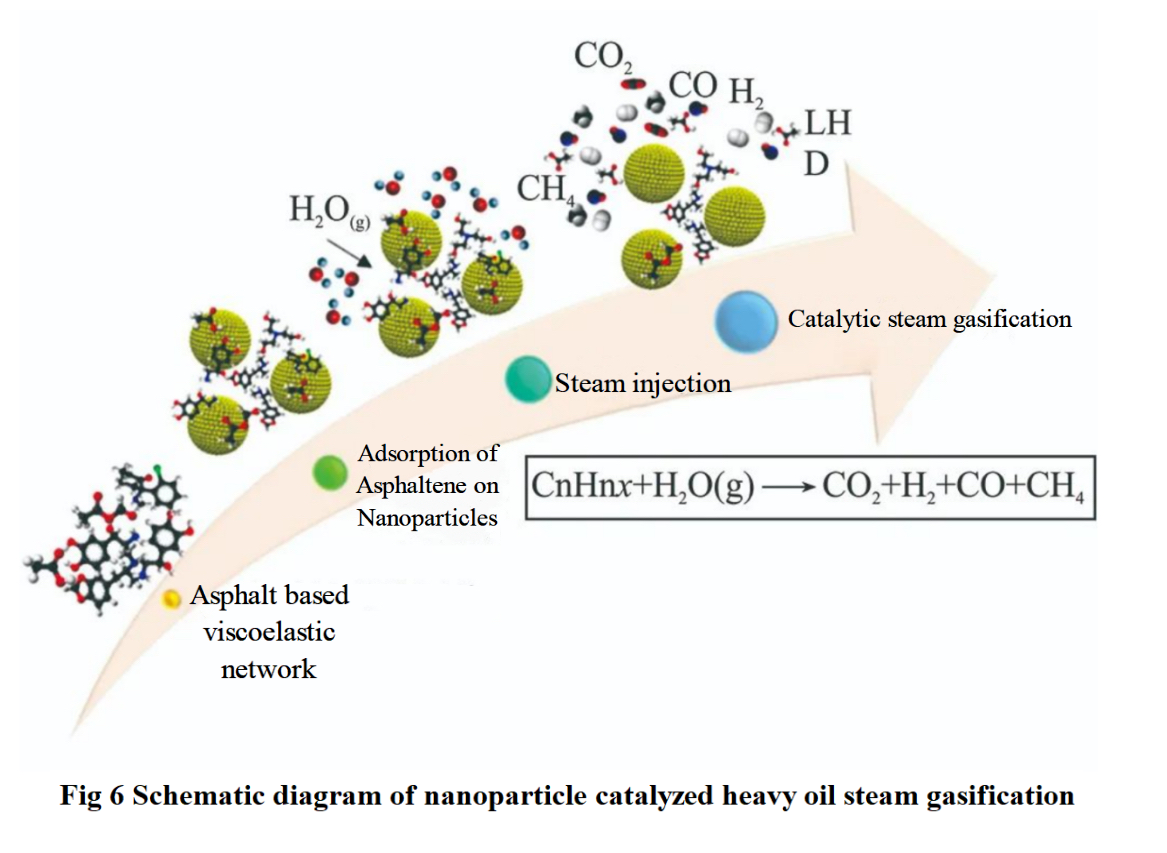
Figure 6 is a schematic diagram of the steam gasification process of heavy oil catalyzed by nanoparticles. Firstly, asphaltene is adsorbed onto nanoparticles and the viscoelastic network disintegrates. Subsequently, the asphaltene material decomposes under steam gasification, and finally the heavy components gasify to produce CO2, H2, CO, CH4, etc. Nassar et al. selected three different types of metal oxide nanoparticles, Fe2O3, Co3O4 and NiO, for heavy oil catalytic-steam viscosity reduction. It was found that Fe2O3, Co3O4, and NiO reduced the reaction temperature from 500℃ to 380℃, 330℃ and 317℃, respectively. Franco et al. used nano SiO2 loaded NiO and PdO catalysts to study the adsorption and steam gasification of asphaltene. The results indicate that 1% Ni and 1% Pd functionalized SiO2 can catalyze the production of more CH4 during the steam gasification process of asphaltene. Rezaei et al. added nano SiO2 and nano g-Fe2O3 during the process of burning oil layers, greatly improving the quality of crude oil and reducing the activation energy of catalytic reactions. Hzashemi et al. dispersed tri metal (W, Ni and Mo) nanoparticles in vacuum diesel and conducted steam assisted gravity drainage (SAGD) experiments, demonstrating that under typical SAGD pressure and temperature conditions, tri metal ultra dispersed nanoparticles can migrate in oil sand media. And the catalytic activity of nanomaterials reduces the production of sulfur-containing and nitrogen-containing gases, reducing greenhouse gas emissions.
In addition, nanomaterials are often used as intelligent nanofluids to improve the recovery rate of heavy oil. Scholars have evaluated the effectiveness of Fe3O4 nanoparticles as a viscosity reducer for heavy oil. When nanoparticles are added separately, the viscosity decreases by 20%. For the ferromagnetic fluid composed of Fe3O4 nanoparticles, lubricating oil, and hexadecyltrimethylammonium bromide (CTAB), the viscosity reduction rates were 81% and 96% at temperatures of 35℃ and 45℃, respectively. The viscosity of heavy oil containing ferromagnetic fluids at 35℃ is close to that of heavy oil without ferromagnetic fluids at 45℃. This reflects that adding ferromagnetic fluids to heavy oil can save thermal energy. Ahoee et al. first studied the role of carbon nanotubes (CNTs) as a viscosity reducer for heavy oil. Using multi walled carbon nanotubes with a diameter of 8-12 nm, different emulsifiers, surfactants, solubilizers, stabilizers, and solvents are added to form different nanofluids. In addition, hydrophilic functionalized nanocomposites (ZnO and CNT) were formed by connecting oleic acid and hexamethylenetetramine (HMTA). The results showed that CNT (E) nanofluid had the best performance, with a 96% reduction in viscosity of heavy oil at 80℃. Functionalized nanocomposites perform better than pure nanocomposites. The optimal viscosity reduction rate of HMTA functionalized nanocomposites in distilled water is 91.6%, while the optimal viscosity reduction rate of oleic acid functionalized nanocomposites in seawater is 93.5%. Researchers believe that hydrophilic functionalization can enhance water solubility, thereby promoting the penetration of nanocomposites into crude oil hydrocarbon chains without aggregation. But the problem with this study is that it did not explore the effect of the base carrier on the viscosity of heavy oil, making the role of CNT in reducing viscosity unclear.
4. Conclusion
1). The cost of emulsification and viscosity reduction is low, the viscosity reduction effect is good, the process is simple, and the effect is fast. However, the universality of emulsifiers for different heavy oils and their own temperature and salt resistance still need to be further improved. How to design and synthesize emulsion viscosity reducing agents with low cost, good effect, and adaptability to different types of heavy oil and high-temperature and high salinity formation environments is an urgent problem that needs to be solved. In the future, in addition to continuously optimizing the synthesis route of anionic - non-ionic composite surfactants and reducing costs, it is also necessary to use computer simulation methods to deeply analyze the interaction between viscosity reducers and heavy oil.
2). Oil soluble viscosity reducer has low energy consumption and can fully contact heavy oil, with great development prospects. However, the viscosity reduction effect is limited and the application cost is high, and the underground mixing conditions have a significant impact. Therefore, further research on the mechanism of viscosity reduction, design and development of efficient and inexpensive oil soluble viscosity reducing agents, and optimization of downhole mixing processes are the focus of research.
3). The combination of hydrothermal cracking and catalytic reactions has made hydrothermal catalytic cracking viscosity reduction technology a promising technology. However, the development of efficient, low-cost, highly active, highly selective, and widely used catalysts is still a long and challenging path. Super dispersed nano catalysts are the focus of future research. In addition, it is also necessary to search for hydrogen donors with high abundance, easy availability, low price, and safety and environmental protection.
4). Nanoparticles have great potential for application as adsorbents and catalysts in medium to deep heavy oil recovery, but this technology is still not mature enough for practical applications. The self aggregation of nanoparticles and the selectivity of heavy oil with different components are its main drawbacks. Secondly, the viscosity reduction mechanism of nanomaterials is still unclear, and further research is needed in the future to determine the optimal reaction conditions and mechanism.
