Research and Application Progress of Nanomaterials in Wellbore Cement for Geological Carbon Dioxide Storage(Part 1)
Abstract
The development of nanotechnology has facilitated the application of nanomaterials in wellbore cement. Nanomaterials possess characteristics of high specific surface area and high reactivity,which can effectively improve the performance of wellbore cement even in small quantities,thus overcoming the limitations of traditional cementitious materials under storage conditions. This article reviews the role of nanomaterials in modifying the wellbore cement,analyzes the three types of nanomaterials,namely oxide nanomaterials,carbon nanomaterials,and nano mineral powders,with emphasis on the mechanisms,modification effects,and application progress of nanomaterials in wellbore cement. Finally,the application of nano reinforced cement-based composites in CO2 geological storage is prospected.
Since the Industrial Revolution, due to the use of a large amount of fossil fuels, the concentration of CO2 in the atmosphere has sharply increased, leading to serious ecological and environmental problems such as climate variability. How to achieve CO2 reduction has become the key to solving the global climate change problem. Global energy related CO2 emissions increased by 0.9% in 2022, exceeding 3.68 Gt. As a developing country, China has successfully fulfilled its commitment to reduce carbon emission intensity and met the requirements of the Paris Agreement. In order to achieve the commitments of peaking carbon emissions by 2030 and achieving carbon neutrality by 2060, carbon capture, utilization, and storage are crucial and necessary.
During geological storage, CO2 leakage may occur during the storage stage or post storage stage. The sealing integrity of cement sheath plays an important role in the effective and safe storage of CO2. In the environment of CO2 geological storage, cementing cement is inevitably subjected to high temperature, high pressure, and CO2 corrosion, leading to carbonation reactions between wellbore cement and concrete, which affects the sealing and mechanical properties of cement.
In recent years, nanomaterials have received widespread attention due to their excellent properties such as high specific surface area and high chemical activity. As a super filling material, their application in cement-based materials has been studied. On the premise of ensuring good dispersion, the addition of nanomaterials can significantly improve the microstructure of cement-based materials and reduce the overall porosity. Nanomaterials can fill pores of 20-150 nm, thereby improving the compactness of cement slurry and interface transition zone, and reducing the migration rate of harmful ions from the outside. At the same time, after the addition of nanomaterials, the cement-based material slurry will also have high compressive and tensile strength, low permeability, and good fluidity, ultimately enabling the nano reinforced cement-based composite material to maintain a certain strength under high temperature and high pressure, reducing the erosion of supercritical CO2.
This article summarizes and discusses the research achievements of nano modified cement-based materials in high temperature, high pressure, and strong corrosion environments both domestically and internationally from the perspective of CO2 geological storage, and looks forward to future research and applications in this field.
1. The Modification Mechanism of Nanomaterials in Cement
When the size of a material changes from macroscopic to nanoscale, its mechanical properties, surface properties, conductivity, chemical reactivity, and optical absorption undergo significant changes. There are three mechanisms for modifying cement-based materials with conventional nanoparticles: 1) .Nanoparticles provide nucleation sites for the hydration process, which can accelerate the initial hydration rate of cement. 2). Nano SiO2 (NS), which has volcanic ash reaction activity, can react with hydration product Ca (OH) 2 to generate more hydrated calcium silicate (xCaO·SiO2·nH2O, C-S-H), playing a role in compacting the slurry structure and improving the properties of cement-based materials. Moreover, due to the high specific surface area of NS, the rate of hydration reaction will also increase. 3). Due to the extremely small size of nanoparticles, they have strong physical filling properties and can greatly improve the microstructure of cement. The first two modification mechanisms occur during the hydration process.
1.1 Hydration Process
The hydration of clinker and mineral admixtures is the basic process that causes cement solidification and hardening. he four main minerals, tricalcium silicate (3CaO·SiO2), dicalcium silicate (2CaO·SiO2), tricalcium aluminate (3CaO·Al2O3), and tetracalcium ferroaluminate (4CaO·Al2O3·Fe2O3), gradually dissolve, and the concentrations of various ions in the aqueous solution continue to accumulate. When the ion concentration in the liquid phase exceeds the solubility of the hydration product and reaches a certain degree of supersaturation, the hydration product begins to form precipitates. The reaction process is shown in equations (1) to (3).
The dissolution process of 3CaO·SiO2:
![]()
The precipitation process of C-S-H:
![]()
The precipitation process of Ca (OH) 2:
![]()
The cement hydration process is shown in Figure 1.
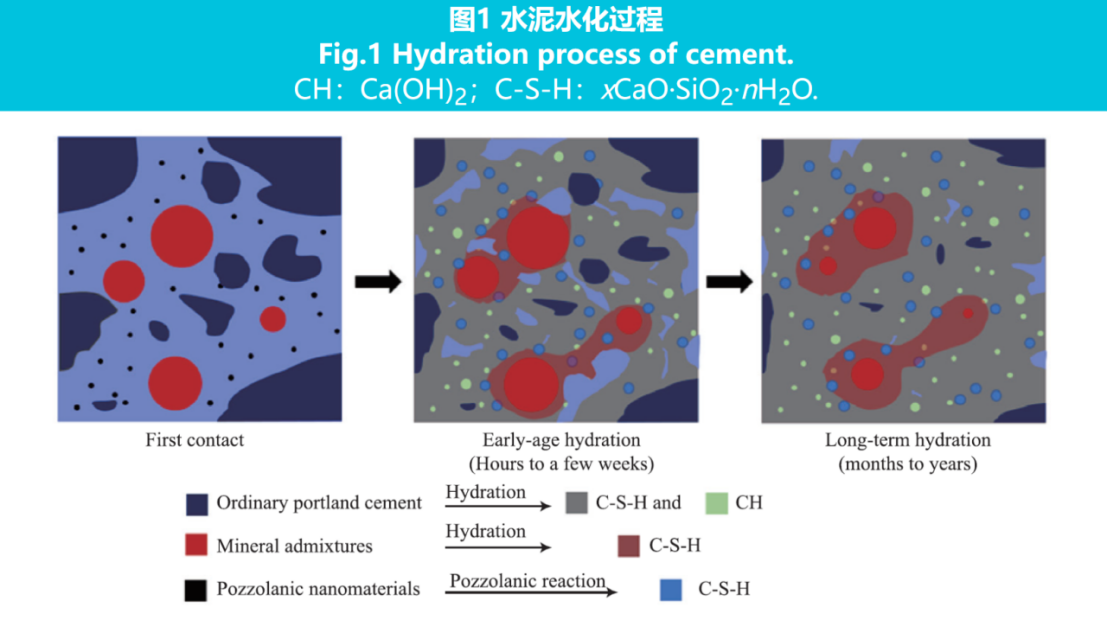
As shown in Figure 1, the hydration of Portland cement generates C-S-H and Ca (OH) 2, leading to a decrease in cement pore content and pore size. The mechanism by which nanoparticles promote cement hydration is that in the early stage of cement hydration, the externally added nanoparticles act as additional nucleation sites, and the cement hydration product C-S-H grows on the surface of the externally added nanoparticles, greatly reducing the nucleation potential and growth surface area of C-S-H. The hydration product C-S-H gel not only grows on the surface of cement particles, but also grows on the surface of a large number of nano particles distributed in the liquid phase of cement slurry, thus accelerating the initial hydration rate of cement and making the spatial distribution of hydration products more uniform.
1.2 Volcanic Ash Reaction
The chemical effect generated by the active components of mineral admixtures in cement, such as active SiO2 and active Al2O3, is called volcanic ash reaction. Among them, active SiO2 can undergo secondary hydration reaction with hydration product Ca (OH) 2 and high alkalinity C-S-H, generating more stable and stronger C-S-H; Active Al2O3 reacts with hydration product Ca (OH)2 to form hydrated calcium aluminate (C-A-H), and then continues to react with gypsum (CaSO4·2H2O) in the cement slurry to form ettringite crystals. The main reaction formula is as follows.
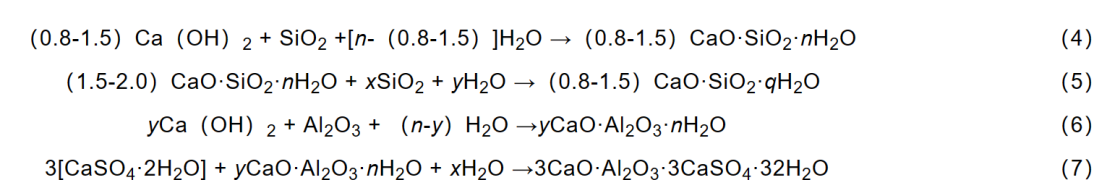
The volcanic ash reaction reduces the content of hydration product Ca (OH) 2, forming additional products such as C-S-H and C-A-H to fill the pores, reducing the porosity of cement and improving its strength and durability. Nanoparticles with volcanic ash reactivity can also react with Ca (OH) 2 to generate additional C-S-H, meaning that mineral admixtures and nanoparticles with volcanic ash reactivity can undergo volcanic ash reaction to generate additional C-S-H. However, nanoparticles have a larger specific surface area, so the volcanic ash reaction rate of NS is 10 times faster than that of silicon powder, and the hydration products are denser in microstructure than those of mineral admixtures.
The action mechanism of nano particles with pozzolanic reactivity is related to their huge specific surface area, which provides additional specific surface area for hydrate deposition and is the nucleation site of C-S-H gel, thus promoting the hydration of cement clinker and the pozzolanic reaction of mineral admixtures, making the cement matrix densified, and ultimately improving the interface transition zone between aggregate and cement paste. Thermodynamically speaking, the driving force of this process mainly comes from the high specific surface energy of the nanoparticles. As the amount of solid phase precipitation on the surface of the nanoparticles increases, the specific surface energy gradually decreases.
1.3 Filling Effect
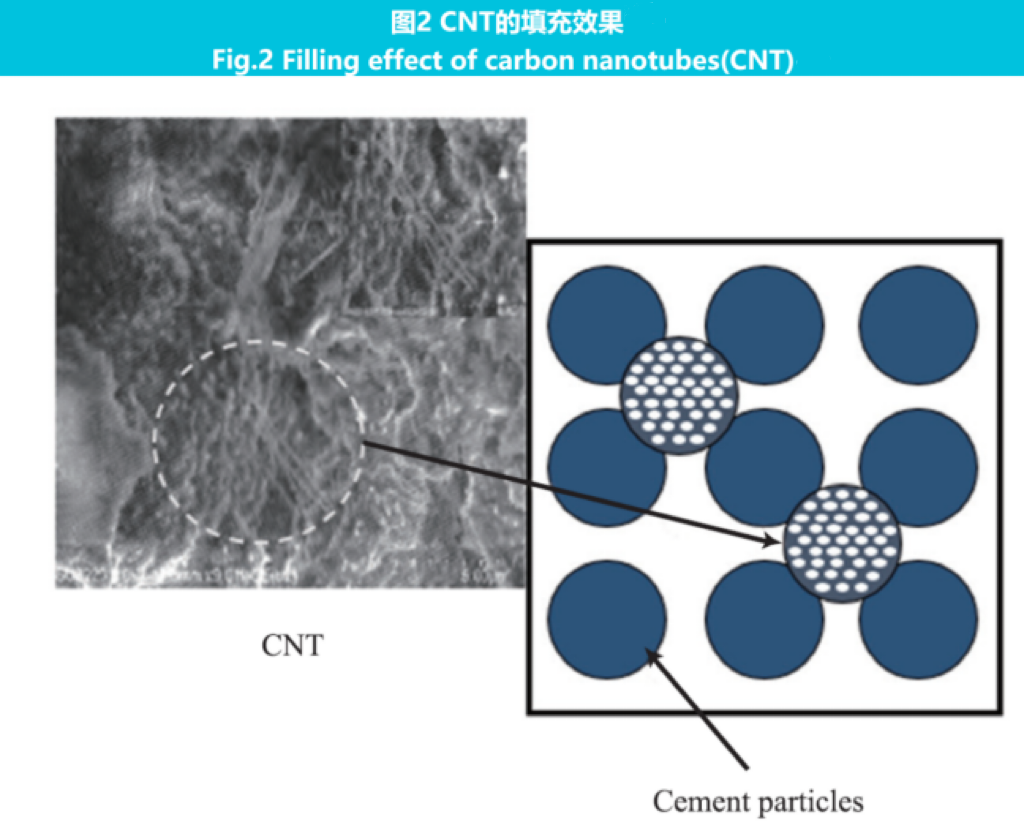
Nanoparticles can fill the pores of cement, improve its microstructure, and macroscopically reduce porosity. When Yu et al. added 4% (w) NS to cement, the porosity decreased to 9.5%. Duan et al. found that after adding 5% (w) nano TiO2 (NT), the structure of cement-based materials became dense, and the nanopore filling effect of NT affected the hydration reaction rate. The pore size distribution of cementing slurry can be changed by adding Al2O3 nanofibers, and the porosity decreases with the addition of nanofibers. In cement paste, pores can generally be divided into two basic size ranges: gel pores (<50 nm) and capillary pores (50-100 nm). After adding nanofibers, compared with the reference sample, the number of pores larger than 100 nm was significantly reduced, and the number of pores between 50-100 nm was significantly increased, effectively refining the pore structure. The filling effect of carbon nanotubes (CNTs) is shown in Figure 2.
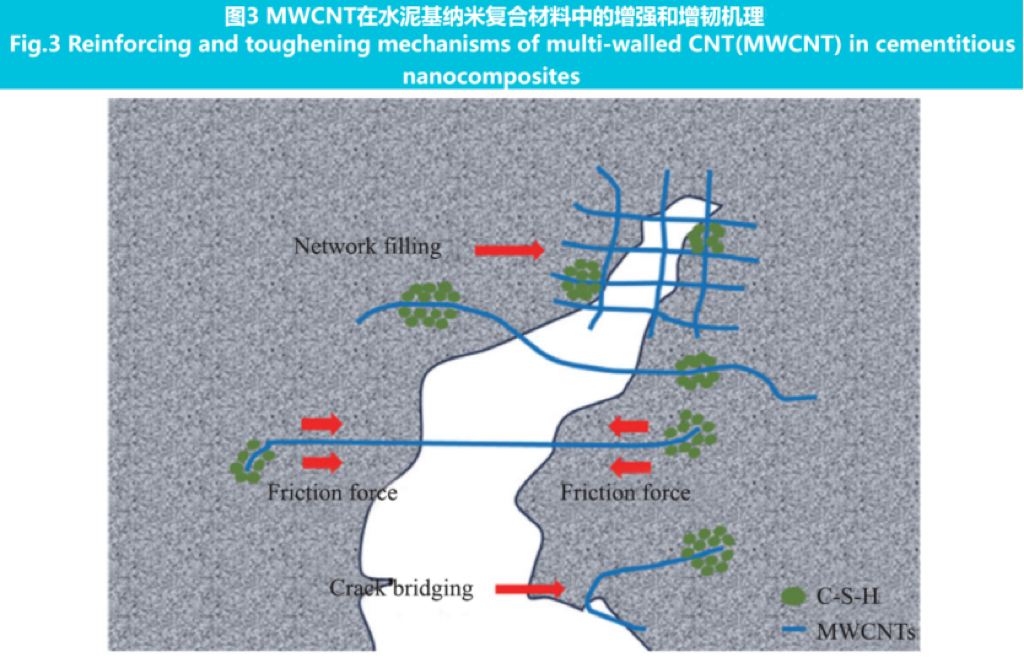
The high aspect ratio characteristics of materials such as carbon nanofibers (CNF) and CNTs enable them to act as physical bridges at the onset of cracks, thereby constraining crack propagation and maintaining structure. CNF and CNT can improve the tensile strength and toughness of cement through bridging effect. The strengthening and toughening mechanism of multi walled CNT (MWCNT) in cement-based nanocomposites is shown in Figure 3.